Abstract
Rheumatoid arthritis (RA) is a joint-related autoimmune disease that is difficult to cure. Most therapeutics act to alleviate the symptoms but not correct the causes of RA. Novel strategies that specifically target the causes are highly needed for RA management. Currently, early interruption of RA is increasingly suggested but the corresponding therapeutics are not available. Vaccines that have shown great success to combat infection, cancer, degenerative diseases, autoimmune diseases, etc. are ideal candidates for a new generation of anti-RA therapeutics to correct the causes and prevent RA or interrupt RA in early phases. Anti-RA vaccines can be divided into two major categories. One is to induce neutralizing antibodies and the other is to induce antigen-specific immune tolerance. The vaccines are inherently linked to nanotechnology because they usually need a biomacromolecule or carrier to provoke sufficient immune responses. In the past decade, designed nanocarriers such as nanoparticles, liposomes, nanoemulsion, etc., have been applied to optimize the vaccines for autoimmune disease treatment. Nanotechnology endows vaccines with a higher biostability, tunable in vivo behavior, better targeting, co-delivery with stimulatory agents, regulatory effects on immune responses, etc. In this review, unmet medical needs for RA treatment and anti-RA vaccinology are first introduced. The development of anti-RA therapies from vaccines to nanovaccines are then reviewed and perspectives on how nanotechnology promotes vaccine development and advancement are finally provided. In addition, challenges for anti-RA vaccine development are summarized and advantages of nanovaccines are analyzed. In conclusion, nanovaccines will be a promising strategy to revolutionize the treatment of RA by correcting the causes in an early phase of RA.
Graphical abstract
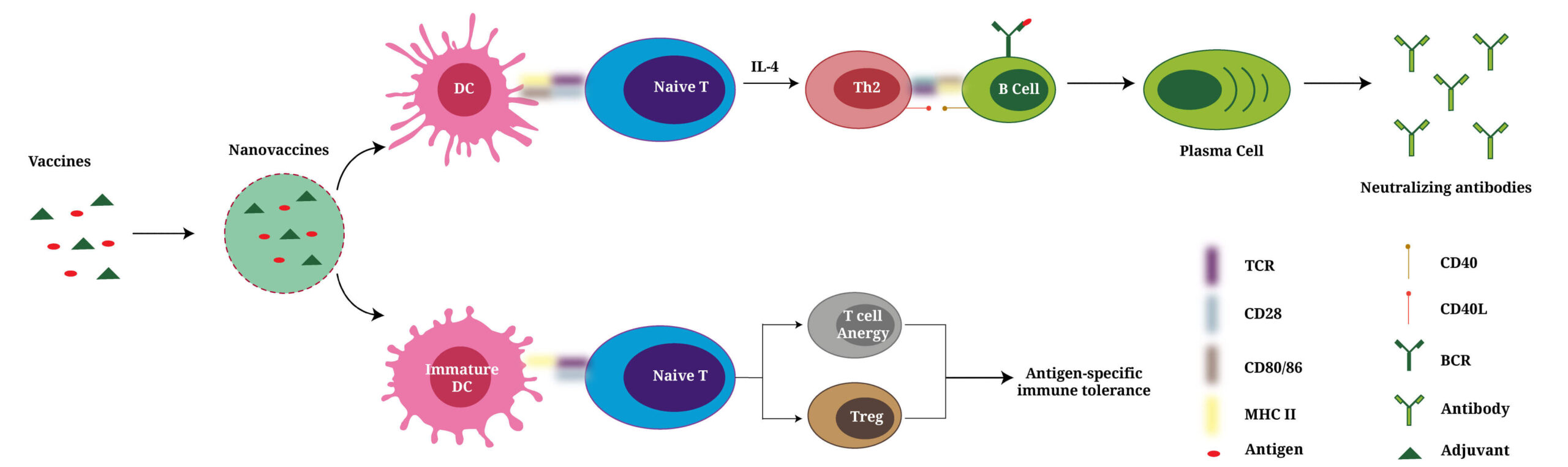
Evolved from vaccines to nanovaccines, they induce either neutralizing antibodies to clear inflammatory factors or antigen-specific immune tolerance to better prevent/treat rheumatoid arthritis.
Introduction
Vaccines are great inventions in the history of human healthcare and have provided great protection to human [1]. They are widely applied and studied to combat infection [2], cancer [3], degenerative diseases [4,5], and autoimmune diseases [[6], [7], [8]]. Among the many diseases, autoimmune diseases are very special because the immune disorders in the diseases add much complexity and uncertainty to the efficacy and safety of vaccines. For example, vaccine administration may aggravate the symptoms of rheumatoid arthritis (RA) in some cases and the application of anti-RA treatment may reduce the efficacy of vaccines in other cases [[9], [10], [11], [12]]. The concerns can be eliminated once the mechanisms of autoimmune disease are elucidated. Unfortunately, for most autoimmune diseases, the mechanisms of their pathogenesis have not been fully elucidated [[13], [14], [15], [16]]. Nevertheless, the largely unknown field has attracted much attention in the past two decades. Many studies have proved the feasibility and efficacy of vaccines and uncover partial mechanisms of vaccines for autoimmune disease treatment [17,18].
RA is a frequently diagnosed autoimmune diseases [19]. Its pathogenesis involves the disorders of immune cells, stromal cells, cytokines, chemokines, etc. (Fig. 1A) [20]. Some anti-RA vaccines have been developed in the past decade. One major type of anti-RA vaccines is anti-cytokine vaccines. This strategy is inspired and adapted from monoclonal antibody-based biologics for RA treatment [21]. Anti-cytokine vaccines can be considered as an advanced therapy compared with monoclonal antibody-based biologics. These anti-cytokine vaccines induce the body to produce antibodies to neutralize proinflammatory cytokines. Compared with in vitro harvested monoclonal antibodies, the anti-cytokine vaccine-induced antibodies are fully humanized and do not need repeated injections, which greatly reduces the risk of cross species contamination, treatment costs, and the induction of anti-drug antibodies in vivo [22,23]. The other strategy for anti-RA vaccine development is the induction of immune tolerance that mainly blunt autoreactive T cells, B cells, etc. [[24], [25], [26]]. Statistically, anti-RA vaccines are much less studied recently compared with that for Multiple sclerosis (MS), another common autoimmune disease [6,7,[27], [28], [29], [30]]. This may be because the antigen for MS has been clearly identified as myelin while the antigens that induce RA are complicated and can be any of the antigens discussed in the later sections. However, many findings from anti-MS vaccines are very inspiring for anti-RA vaccine studies. For example, Krienke C et al. have recently published the first mRNA vaccine for MS treatment. They used MS-related autoantigen encoding mRNA to develop vaccines. Antigen presenting cells (APCs) took and presented the autoantigens in vaccines to T cells without costimulatory signals, which blunted autoreactive T cells to the MS-related autoantigens. This inspires that anti-RA vaccines may also be able to enter a mRNA vaccine era.
Back to two decades ago, the period that nanotechnology has not been in full bloom like nowadays for medical applications, vaccines inherently utilize and link to the concept of nanotechnology. This is because nature protein-based antigens usually hind their epitopes inside the structure and epitope-based antigens are too small in size to induce a sufficient immune response [31,32]. In both cases, antigens need to be linked or carried by a larger and more immunogenic carrier to stimulate immune system and achieve therapeutic effects. To date, nanocarriers not only can deliver antigens more efficiently but also modulate the immune responses caused by antigens [2,3,33,34]. Therefore, nanocarriers are equally or even more important for the vaccine development compared with the antigens. The advancements of nanocarriers will greatly promote the progress of vaccines.
(Nano)vaccine advancements for RA treatment has not been reviewed recently. To learn from the past and lead a revolution for future, this review will comprehensively introduce the treatment options and unmet medical needs for RA, the merits and mechanisms of (nano)vaccines for RA treatment, the (nano)vaccines developed for RA treatment, and future perspectives to develop more efficient and safer (nano)vaccines for RA treatment.
Section snippets
Unmet medical needs of RA treatment
RA pathogenesis can be understood from different aspects such as inflammation [35], autoimmunity [36], proliferation of synovial tissues [37], etc., which are corresponding to different treatments. For instance, Non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids (GCs) act to alleviate inflammation. Methotrexate (MTX) inhibits the proliferation of synovial tissues. Immunosuppressants like Cyclosporine A (CyA) work to modulate autoimmunity. Current RA therapies are standardized by
Vaccinology for RA treatment
Self-reactive T cells and B cells are two subtypes of lymphocytes in healthy individuals [[51], [52], [53]]. In normal cases, they are presented in the periphery and regulated by peripheral tolerance mechanisms that include anergy, exhaustion, lack of tissue homing, and regulatory subpopulations of T cells and B cells. In RA patients, the immune tolerance is impaired. Either the negative selection process of self-reactive T cells is impaired or the self-antigens are presented to T cells
Vaccines for RA treatment
Many powerful vaccines have been made empirically in history. Modern advancement in immunology have provided tremendous immunological knowledge for vaccinology, which has significantly changed nowadays vaccine design [55]. Vaccines for RA treatment can be composed of recombinant proteins, peptides, or nucleic acids, and act to induce antigen-specific immune tolerance or anti-cytokine neutralizing antibodies (Table 1).
Challenges for the development of vaccines for RA
Vaccines for autoimmune disease treatment are much less studied compared with anti-infective and anti-tumor vaccines. Different from the fact that many success anti-infective vaccines used inactivated pathogens, most autoimmune disease vaccines are composed of specific antigenic epitopes. These epitopes are highly immunogenic and easy to produce, which makes them attractive candidates for future vaccines. However, many such vaccines failed when they were transferred from animal models to human.
Advantages of nanovaccines
Nanotherapeutics have many advantages for the treatment of autoimmune diseases and have been comprehensively reviewed recently in several articles [17,63,97]. As a major type of nanotherapeutics, nanovaccines are composed of antigens or antigen-encoding agents, immunomodulatory agents, etc. loaded in nanocarriers. The nanocarriers for nanovaccines can be divided into two major categories by functions, nanocarriers for delivery only, and nanocarriers for delivery and immune modulations. Most
Protein nanovaccines
An early study by Kim W et al. fabricated a Type II collagen (CII) encapsulated PLGA nanoparticles. The PLGA-CII vaccine was given orally. One single administration of the vaccine significantly reduced CIA incidence and severity, anti-CII antibodies in serum, and the proliferation of CII-specific T cell. This is because the PLGA-CII sustainably released CII over one month, which diminished the need for immunization boosters. PLGA-CII showed a significantly higher efficacy than free CII
Other nanovaccines maybe potentially inspirable for RA treatment
The above summarized (nano)vaccines are developed to blunt immune responses or produce neutralizing antibodies, which reduce inflammation and protect tissue damages in RA. However, these studies barely mention pain management that is also critical for RA treatment. Von Loga I et al. developed a nanovaccine for chronic pain management. Although they conducted the study in a murine osteoarthritis (OA) model, it is instructive to manage the chronic pain in RA. They coupled Nerve growth factor
Summary and outlook
Most vaccines are developed to combat infections and cancers. Actually, vaccines are inherently powerful therapeutics for the treatment of autoimmune diseases because their great capacity to remodel the immune system. Different from other diseases that need great promotion of immune responses, the aim of the vaccines for autoimmune diseases is a selective suppression of immune responses. For RA, a common autoimmune disease, continuous immune stimulation is the main contributor to disease
Acknowledgement
We sincerely thank the support from the Natural Science Foundation of Henan Province (No. 202300410419) and the Key Project of the Ministry of Education of Henan Province for Higher Education Institutions, China (No. 21A350015). The authors thank Steven A. Goldstein Collegiate Professor Dr. Lonnie Shea from University of Michigan for his very precious and instructive opinions.
References:
Nan Zhang, Mengru Li, Ziye Hou, Lan Ma, Ayesha Younas, Ziyi Wang, Xinchi Jiang, Jianqing Gao
a] Department of Pharmaceutics, School of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450001, Henan, PR China.
b] Institute of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, Zhejiang, PR China.
c] Key Laboratory of Targeting Therapy and Diagnosis for Critical Diseases, Henan Province, Zhengzhou 450001, Henan, PR China.
d] College of Pharmacy, Inner Mongolia Medical University, Jinshan Development Zone, Hohhot 010110, Inner Mongolia, PR China.
1. D. Prosperi et al. Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases Semin. Immunol. (2017).
2. T. Mamo et al. Nanovaccinology: the next generation of vaccines meets 21st century materials science and engineering Vaccine (2012).
3. A. Sidorov et al. Fc fragments of immunoglobulin G are an inductor of regulatory rheumatoid factor and a promising therapeutic agent for rheumatic diseases Int. J. Biol. Macromol. (2017).
4. K. Mikecz et al. An epitope-specific DerG-PG70 LEAPS vaccine modulates T cell responses and suppresses arthritis progression in two related murine models of rheumatoid arthritis Vaccine (2017).
5. Y. Sawaguchi et al. Suppression of murine collagen-induced arthritis by vaccination of synovial vascular endothelial cells Life Sci. (2013).
6. R.A. Ratsimandresy et al. Active immunization against IL-23p19 improves experimental arthritis Vaccine (2011).
7. T. Wu et al. A novel recombinant RANKL vaccine prepared by incorporation of an unnatural amino acid into RANKL and its preventive effect in a murine model of collagen-induced arthritis Int. Immunopharmacol. (2018).
8. H. Yuan et al. Therapeutic role of a vaccine targeting RANKL and TNF-α on collagen-induced arthritis Biomaterials (2012).
9. O. Pala et al. B lymphocytes in rheumatoid arthritis and the effects of anti-TNF-α agents on B lymphocytes: a review of the literature Clin. Ther. (2018).
10. N. Zhang et al. Selective inhibition of tumor necrosis factor receptor-1 (TNFR1) for the treatment of autoimmune diseases Cytokine Growth Factor Rev. (2020).
11. X. Zhao et al. Different protective efficacies of a novel antigen-specific DNA vaccine encoding chicken type collagen via intramuscular, subcutaneous, and intravenous vaccination against experimental rheumatoid arthritis Biomed. Pharmacother. (2021).
12. D.H. Zimmerman et al. CEL-2000: A therapeutic vaccine for rheumatoid arthritis arrests disease development and alters serum cytokine/chemokine patterns in the bovine collagen type II induced arthritis in the DBA mouse model Int. Immunopharmacol. (2010).
13. M.E.M. El Shikh et al. Extracellular traps and PAD4 released by macrophages induce citrullination and auto-antibody production in autoimmune arthritis J. Autoimmun. (2019).
14. N. Zhang et al. Recent advances in the development of vaccines for chronic inflammatory autoimmune diseases Vaccine (2018).
15. L.M. Casey et al. Conjugation of transforming growth factor beta to antigen-loaded poly(lactide- co-glycolide) nanoparticles enhances efficiency of antigen-specific tolerance Bioconjug. Chem. (2018).
16. L.J. O’Neil et al. Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease Trends Mol. Med. (2019).
17. S. Khatri et al. Cyclic citrullinated peptide aptamer treatment attenuates collagen-induced arthritis Biomacromolecules (2022).
18. D.R. Wilson et al. Synthesis and evaluation of cyclosporine A-loaded polysialic acid-polycaprolactone micelles for rheumatoid arthritis Eur. J. Pharm. Sci. (2014).
19. P. Conigliaro et al. Challenges in the treatment of rheumatoid arthritis Autoimmun. Rev. (2019).
20. J.S. Smolen et al. Rheumatoid arthritis Lancet (2016).
21. E. Neumann et al. Rheumatoid arthritis progression mediated by activated synovial fibroblasts Trends Mol. Med. (2010).
22. G.S. Firestein et al. Immunopathogenesis of rheumatoid arthritis Immunity (2017).
23. C. Peres et al. Preclinical models and technologies to advance nanovaccine development Adv. Drug Deliv. Rev. (2021).
24. T. Zaheer et al. Topical review on nano-vaccinology: biochemical promises and key challenges Process Biochem. (2021).
25. K.L. Hess et al. Polyplexes assembled from self-peptides and regulatory nucleic acids blunt toll-like receptor signaling to combat autoimmunity Biomaterials (2017).
26. G. Cappellano et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis Vaccine (2014).
27. L.H. Tostanoski et al. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific Cell Rep. (2016).
28. R. Kuo et al. Peptide-conjugated nanoparticles reduce positive co-stimulatory expression and T cell activity to induce tolerance Mol. Ther. (2017).
29. A. Page et al. Antigen-specific tolerance approach for rheumatoid arthritis: past, present and future Joint Bone Spine (2021).
30. T. Jia et al. Strategies for active TNF-α vaccination in rheumatoid arthritis treatment Vaccine (2013).
31. G.R. Burmester et al. Novel treatment strategies in rheumatoid arthritis Lancet (2017).
32. I.B. McInnes et al. Pathogenetic insights from the treatment of rheumatoid arthritis Lancet (2017).
33. P. Serra et al. Nanoparticle-based autoimmune disease therapy Clin. Immunol. (2015).
34. T. Su et al. Polymer nanotherapeutics to correct autoimmunity J. Control. Release (2022).
35. C.S. Curran et al. PD-1 immunobiology in systemic lupus erythematosus J. Autoimmun. (2019).
36. J.M. Berthelot et al. Trained immunity and autoimmune disease: did eve sin before Adam? Joint Bone Spine (2019).
37. D. Alvarez-Sierra et al. Analysis of the PD-1/PD-L1 axis in human autoimmune thyroid disease: insights into pathogenesis and clues to immunotherapy associated thyroid autoimmunity J. Autoimmun. (2019).
38. L.E. Guimarães et al. Vaccines, adjuvants and autoimmunity Pharmacol. Res. (2015).
39. A. Carambia et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice J. Hepatol. (2015).
40. D.P. McCarthy et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy Nanomedicine (2017).
41. M. Schwartz et al. A common vaccine for fighting neurodegenerative disorders: recharging immunity for homeostasis Trends Pharmacol. Sci. (2004).
42. S. Shah et al. Nanomedicine based approaches for combating viral infections J. Control. Release (2021).
43. B. Pulendran et al. The science and medicine of human immunology Science (2020).
44. Y. Zhang et al. Nanovaccines for cancer immunotherapy Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. (2019).
45. M. Romero-Ramos et al. Vaccination strategies for Parkinson disease: induction of a swift attack or raising tolerance? Hum. Vaccin. Immunother. (2014).
46. Z. Hunter et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease ACS Nano (2014).
47. M.A. Friedman et al. Vaccinations for rheumatoid arthritis Curr. Opin. Rheumatol. (2016).
48. M. Crnkic Kapetanovic et al. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis Arthritis Res. Ther. (2013).
49. K.L. Winthrop et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis Ann. Rheum. Dis. (2016).
50. L.S. Yun et al. Polymeric micelles for the treatment of rheumatoid arthritis Crit. Rev. Ther. Drug Carrier Syst. (2019).