Introduction
There is a considerable imbalance between the demand and supply of donor organs worldwide. Organ preservation is a prerequisite for an urgent increase in the availability of organs for solid organ transplantation (SOT). An ideal organ preservation technique should not only preserve isolated organs but also offer the possibility of rehabilitation and evaluation of organ function prior to transplantation.
The most widely used donor organ preservation technique is cold static preservation (CSP). Machine perfusion (MP) has shown its advantages over conventional methods in many aspects. Mesenchymal stromal cells (MSCs) are a type of multipotent progenitor cells with a spindle shape and ability to differentiate into chondrocytes, osteoblasts and adipocytes.
MSCs can readily access the microvessels of isolated organs using this approach, allowing an adequate number of cells to settle in the local region. Ex vivo preservation, on the other hand, generates a milieu free of allogeneic immune attack, which favours MSC survival and function. These breakthroughs allow for the full utilisation of MSC potential during ex vivo preservation, transforming organ preservation from passive storage to active preconditioning and accelerative resuscitation. This review provides an integral and updated view of the current understanding of the combination of MSC’s with MP for organ preservation.
Theoretical basis of combining mesenchymal stromal cells with machine perfusion as a novel organ preservation strategy
Quality of donor organs is a major factor for rapid function resumption after transplantation and one of the key issues affecting graft quality is (Ischemia/reperfusion) I/R injury. I/R injury is an inevitable process in SOT. Strong evidence suggests that MSCs have a potential ability to alleviate I/R injury in various organs, including the heart, kidneys, liver, brain and lungs. This evidence provides a theoretical basis for combining MSCs with MP as a novel organ preservation strategy to improve graft quality in SOT.
In the following section, we discuss a few specific mechanisms underlying the protective effects of MSCs on I/R-injured organs
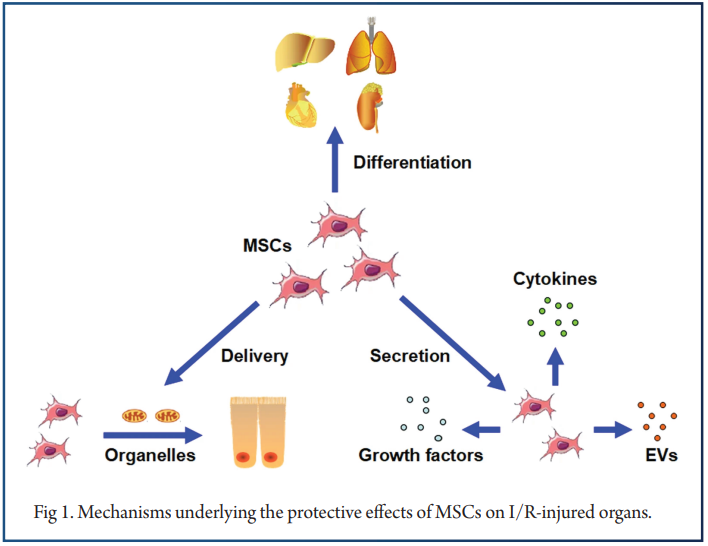
Lastly, MSCs exhibit the ability to directly deliver organelles to injured cells, thereby contributing to survival and proliferation.Based on multiple actions, MSCs can alleviate the I/R injury on target tissues. First, MSCs can differentiate into related functional cells, thus favoring regeneration. Second, via secretion of various cytokines, growth factors and EVs (Extracellular vesicles), MSCs can generate a better post-injury microenvironment.
Recent studies have indicated that the regenerative capacity of MSCs, especially on the function of some important organelles, may be fulfilled by a direct organelle delivery approach. Compared with modulation by differentiation or paracrine actions that activate downstream effectors, direct organelle delivery is a more efficient and economical strategy. It helps recipient cells expend less time and energy on synthesis of related organelles, which is important in critical situations.
Experimental data on combination of mesenchymal stromal cells and machine perfusion in ex vivo resuscitation of donated organs
The evidence mentioned above makes MSCs ideal candidates for ex vivo cell-based therapy in organ preservation. In recent years, the combination of MSCs and MP in ex vivo resuscitation of donated organs was attempted in the kidneys, lungs and liver and some encouraging results were obtained.
MSCs in renal recovery
Pool et al. found that after 7 hours of Normothermic Machine Perfusion (NMP) with MSCs, a tiny proportion of human MSCs could be detected in the lumen of glomerular kidney capillaries. Furthermore, the retained MSCs were discovered to be structurally intact, implying that their potential biological activity could be preserved during perfusion.
Gregorini et al. conducted a series of experiments. Donated kidneys were first subjected to a 20-min period of warm ischemia and then were preserved with continuous perfusion at 4 °C. In the experimental group, MSC-EVs were added to the Hypothermic MP (HMP). MSCs were isolated from rats that highly expressed enhanced green fluorescence protein (EGFP). In the control group, only HMP was administered.
After 4 h of perfusion, the kidneys were retrieved and the effluent fluid was collected for further analysis. EGFP staining showed that MSCs were successfully located in the vessels, tubules and interstitium, without signs of embolism in macrovessels/microvessels. Histologically, renal ischemia damage was ameliorated in kidneys perfused with MSC-EVs compared with that in the control group.
At the gene level, the experimental group presented with multiple up-regulated genes, mainly related to cell energy metabolism and membrane transport. The authors also analyzed some markers of ischemia damage in the effluent fluid. The levels of injury markers such as lactic dehydrogenase and lactate were significantly lower in the experimental group than those in the control group, in parallel with the change in malondialdehyde (MDA) levels, which is an oxidative stress indicator.
The effluent glucose level was lower in the MSC-EV group, whereas pyruvate levels showed the opposite trend. The authors concluded that via re-balancing of energy metabolism enzymatic machinery, which is vital for cell viability, the addition of MSCs during HMP protected donated organ injury.
MSCs in lung function restoration
Lung graft acceptance rate remains the lowest of any SOT. It is estimated that only 15-25% of donated lungs meet the selection criteria and are finally transplanted. In order to increase suitability of lungs for clinical utilization, McAuley et al. collected human lungs from brain-dead donors that had been rejected for transplantation and were preserved in an ex vivo lung perfusion (EVLP) container at 36 °C.
Thereafter, they determined the level of alveolar fluid clearance (AFC) in the preserved lung lobes. The AFC level represents the capacity to reabsorb alveolar edema fluid and is associated with good clinical outcomes post-transplantation. If the AFC level was < 10% per hour, an indicator of poor prognosis, MSCs were administered directly into the perfusate in the experimental group and were continuously perfused for 4 h.
The addition of MSCs significantly increased AFC levels in the experimental group compared with those of the control group, which was preserved with perfusion only. Their study proved that the combination of MSCs with Subnormothermic MP (SNMP) was effective in restoring the function of donor lungs and was able to expand the donor pool by utilizing non transplantable organs. However, the former study did not focus on alterations in lung function or overall lung fluid balance.
To investigate this aspect, Stone et al. administered human umbilical cord-derived MSCs (UC-MSCs) into the perfusate of a mouse donation after circulatory death (DCD) lung preservation model. Improved lung compliance, pulmonary arterial pressure (PAP), lung weight and neutrophil infiltration were observed in the lungs supplemented with MSCs.
MSCs in liver function preservation
Yang et al. explored the combination of MSCs with NMP on liver preservation. Rat liver was subjected to a 30-min period of warm ischemia and then harvested to establish a rat liver model. NMP was performed continuously for 8 h during ex vivo preservation.
In the combination group, Bone marrow-derived MSCs (BM-MSCs) were injected via the portal vein immediately after the system was connected, whereas in the control group, only NMP was administered. They found that the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were significantly lower in the combination group, thereby indicating better preservation of liver function.
The combination of MSCs with NMP resulted in the reduction of the levels of Myeloperoxidase (MPO) and Malondialdehyde (MDA) and mitochondrial damage; increased the production of Glutathione (GSH); and ameliorated DCD liver oxidative stress injury.
Conclusion and future perspectives
In conclusion, encouraging experimental data suggest that the innovative organ preservation strategy of combining MSCs with MP creates a bridge between organ procurement and transplantation during which I/R injury can be significantly decreased and graft function can be preserved.
This method allows for a more well-planned line of action during the entire perioperative period. Instead of immediately considering medical measures and minimising ischemia time through rash decision-making, clinicians can prepare and proceed at the optimal time.
References
- Abecassis M, Bartlett ST, Collins AJ et al (2008) Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidni Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol 3(2):471–480
- Bae C, Henry SD, Guarrera JV (2012) Is extracorporeal hypothermic machine perfusion of the liver better than the “good old icebox.” Curr Opin Organ Transplant 17(2):137–142
- Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G (2012) Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant 27(8):3037–3042
- Bon D, Chatauret N, Giraud S, Thuillier R, Favreau F, Hauet T (2012) New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol 8(6):339–347
- Brasile L, Henry N, Orlando G, Stubenitsky B (2019) Potentiating renal regeneration using mesenchymal stem cells. Transplantation 103(2):307–313
- Brasile L, Stubenitsky BM, Booster MH et al (2002) Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation 73(6):897–901
- Braza F, Brouard S, Chadban S, Goldstein DR (2016) Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol 12(5):281–290
- Cantaluppi V, Gatti S, Medica D et al (2012) Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microR- NA-dependent reprogramming of resident renal cells. Kidney Int 82(4):412–427
- Cao H, Yang L, Hou B, Sun D, Lin L, Song HL, Shen ZY (2020) Heme oxygenase-1-modified bone marrow mesenchymal stem cells combined with normothermic machine perfusion to protect donation after circulatory death liver grafts. Stem Cell Res Ther 11:218
- Collino F, Bruno S, Incarnato D et al (2015) AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying MicroRNAs. J Am Soc Nephrol 26(10):2349–2360





