Introduction
Pure red cell aplasia (PRCA) is a rare but serious complication occurring in 7–10% of patients after major ABO-mismatched allogeneic stem cell transplantation (HSCT), often requiring treatment beyond standard supportive care. Though some cases resolve within 3–6 months, therapies like erythropoietin, plasmapheresis, immunosuppressants, and TPO-mimetics have shown limited success.
Daratumumab, an anti-CD38 monoclonal antibody, has recently shown promising and rapid results in PRCA, even after other treatments fail. In a reported case of PRCA with mixed chimerism following HSCT for aplastic anemia, daratumumab led to quick transfusion independence after the first dose, despite delays due to infections. This case supports early use of daratumumab to avoid ineffective therapies and reduce risks of graft failure and immunosuppression-related complications.
Case Presentation
In October 2023, a 29-year-old woman presented with fatigue and bleeding symptoms and was diagnosed with aplastic anemia (AA) based on pancytopenia and a hypocellular bone marrow. Virology tests and cytogenetic studies were unremarkable, though next-generation sequencing revealed an EZH2 mutation. She underwent allogeneic hematopoietic stem cell transplantation (HSCT) in January 2024 from an HLA-identical sibling with a major ABO mismatch (recipient O+, donor A+). The conditioning regimen included fludarabine and cyclophosphamide, with GvHD prophylaxis comprising thymoglobulin, methotrexate, and cyclosporine. Due to a high anti-A isohemagglutinin titer, plasmapheresis was performed before stem cell infusion.
Following transplant, mixed chimerism (MC) was observed, and while neutrophil and platelet engraftment occurred by day +35, red cell recovery was absent. Weekly transfusions and erythropoietin were initiated, but after three months, bone marrow studies confirmed pure red cell aplasia (PRCA). Rituximab was administered without benefit, and the patient remained transfusion-dependent with elevated ferritin levels requiring iron chelation.
Due to the failure of standard treatments, daratumumab (an anti-CD38 monoclonal antibody) was started in September 2024. After an initial delay due to mild infections, the patient completed four doses without infusion-related reactions. Although she experienced CMV reactivation, it was successfully treated with valganciclovir. One month post-treatment, she achieved transfusion independence, her hemoglobin stabilized at 12 g/dL, anti-A antibodies were undetectable, and her blood group converted to donor type (A+). Mixed chimerism persisted, but her clinical and hematologic outcomes markedly improved, supporting the early use of daratumumab as an effective therapy in post-transplant PRCA. Figure 1 summarizes the hemoglobin, reticulocytes, and titer anti-A trend.
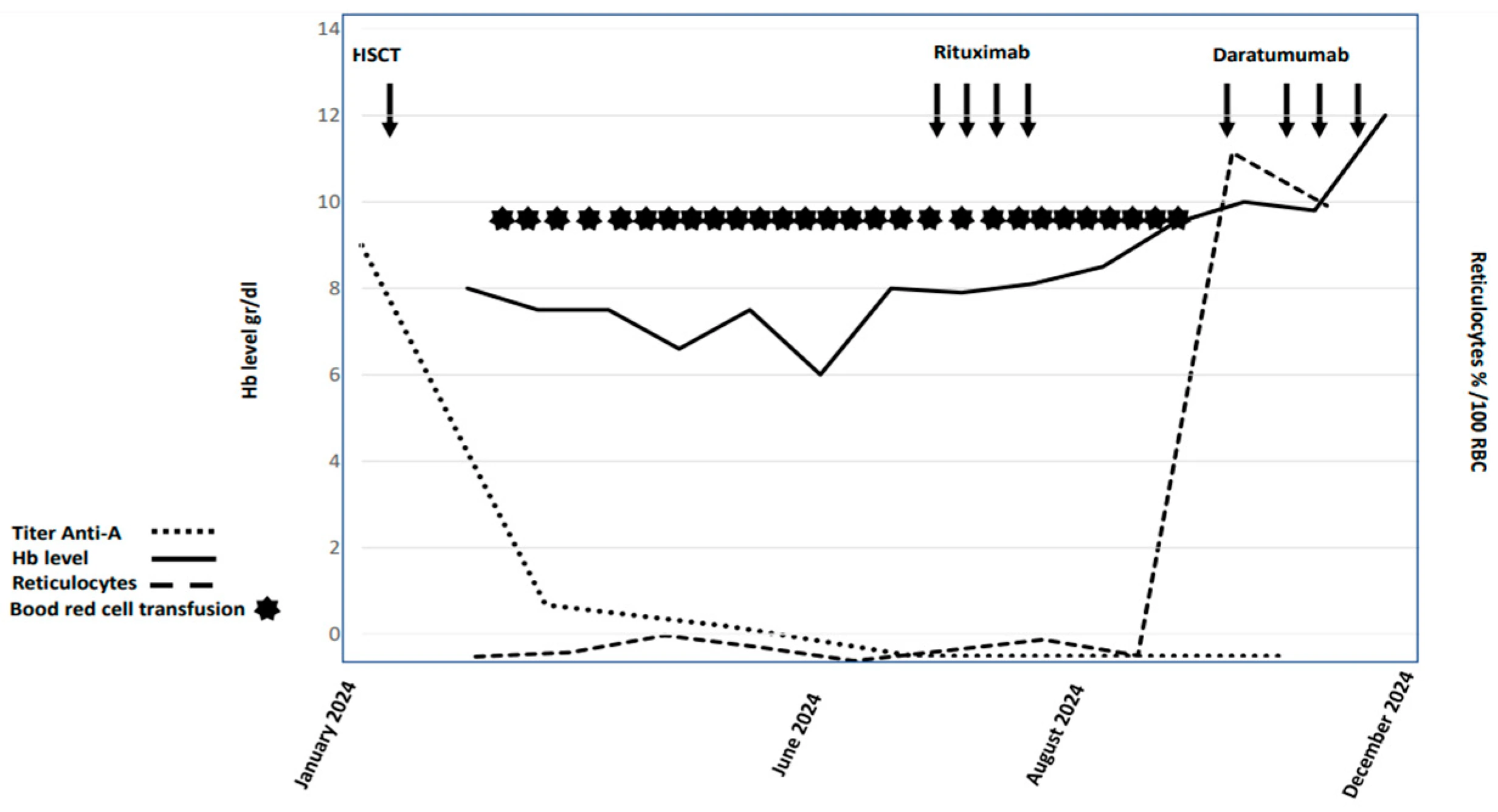
Figure 1. Graphic summary of hemoglobin and reticulocytosis trend and anti-A titer of the patient.
Conclusion
Pure red cell aplasia (PRCA) and mixed chimerism (MC) are two complications that may arise following allogeneic hematopoietic stem cell transplantation (HSCT), particularly in patients with aplastic anemia (AA), and are associated with an increased risk of graft failure (GF). While MC is not synonymous with GF, it may persist in non-malignant conditions due to immune tolerance between donor and recipient. Factors such as sex mismatch (male donor, female recipient) and conditioning regimens using cyclophosphamide plus ATG are linked to a higher risk of GF, whereas fludarabine-based protocols with low-dose cyclophosphamide may reduce this risk. In the context of PRCA, which is often related to high isoagglutinin titers and ABO mismatch, daratumumab has demonstrated rapid and sustained efficacy in reducing transfusion dependence by eliminating residual host plasma cells, especially when used early (within 3–6 months post-HSCT).
Daratumumab, a CD38-targeting monoclonal antibody, has emerged as a safe and effective treatment for PRCA, particularly in cases where conventional immunosuppressive therapies like rituximab are ineffective. Early use of daratumumab can alleviate transfusion burdens, lower the risk of iron overload, and minimize the adverse effects of prolonged immunosuppression, including infections. Although long-term data are limited, existing evidence—including rapid hematologic recovery and reduced anti-A antibody titers—supports its integration into early post-HSCT management strategies. Further clinical studies are warranted to evaluate long-term outcomes and explore alternative anti-CD38 agents, such as isatuximab, for optimizing care in this high-risk patient population.
References
1. Longval, T.; Galimard, J.; Leprêtre, A.; Suarez, F.; Amiranoff, D.; Cazaux, M.; Kaphan, E.; Michonneau, D.; Dhedin, N.; Coman, T.; et al. Treatment for pure red cell aplasia after major ABO-incompatible allogeneic stem cell transplantation: A multicentre study. Br. J. Haematol. 2021, 193, 814–826.
2. Hirokawa, M.; Fukuda, T.; Ohashi, K.; Hidaka, M.; Ichinohe, T.; Iwato, K.; Kanamori, H.; Murata, M.; Sakura, T.; Imamura, M.; et al. Efficacy and long-term outcome of treatment for pure red cell aplasia after allogeneic stem cell transplantation from major ABO-incompatible donors. Biol. Blood Marrow Transplant. 2013, 19, 1026–1032.
3. Griffith, L.M.; McCoy, J.P.; Bolan, C.D.; Stroncek, D.F.; Pickett, A.C.; Linton, G.F.; Lundqvist, A.; Srinivasan, R.; Leitman, S.F.; Childs, R.W. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative haematopoietic cell transplantation. Br. J. Haematol. 2005, 128, 668–675.
4. Chapuy, C.I.; Kaufman, R.M.; Alyea, E.P.; Connors, J.M. Daratumumab for delayed red-cell engraftment after allogeneic transplantation. N. Engl. J. Med. 2018, 379, 1846–1850.
5. Bathini, S.; Holtzman, N.G.; Koka, R.; Singh, Z.; Wilding, E.; Zou, Y.; Ruehle, K.; Kocoglu, M.H.; Badros, A.; Hardy, N.; et al. Refractory postallogeneic stem cell transplant pure red cell aplasia in remission after treatment with daratumumab. Am. J. Hematol. 2019, 94, E216–E219.
6. Metafuni, E.; Barreto, M.T.B.; Valentini, C.G.; Giammarco, S.; Limongiello, M.A.; Sorà, F.; Bianchi, M.; Massini, G.; Piccirillo, N.; Putzulu, R.; et al. Pure red cell aplasia among ABO mismatched hematopoietic stem cell transplant recipients: A 13-years retrospective study and literature review. Front. Oncol. 2024, 14, 1386670.
7. Salas, M.Q.; Alahmari, A.; Lipton, J.H. Successful treatment of refractory red cell aplasia after allogeneic hematopoietic cell transplantation with daratumumab. Eur. J. Haematol. 2020, 104, 145–147.
8. Rautenberg, C.; Kaivers, J.; Germing, U.; Haas, R.; Ackerstaff, S.; Hoffmann, T.; Kobbe, G.; Schroeder, T. Daratumumab for treatment of pure red cell aplasia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2020, 55, 1191–1193.
9. Henig, I.; Yehudai-Ofir, D.; Zohar, Y.; Zuckerman, T. Pure Red cell aplasia following ABO-mismatched allogeneic hematopoietic stem cell transplantation: Resolution with daratumumab treatment. Acta Haematol. 2021, 144, 683–687.
10. Shamshad, G.U.; Ahmed, S.; Bhatti, F.A.; Ali, N. Mixed donor chimerism in non-malignant haematological diseases after allogeneic bone marrow transplantation. J. Coll. Physicians Surg. Pak. 2012, 22, 765–768.