Introduction
Kidney transplantation remains the preferred treatment for end-stage kidney disease, offering superior survival and quality of life compared to dialysis. Advances such as broader organ sharing, paired donor exchanges, and the use of marginal kidneys have expanded transplant opportunities. These achievements are largely due to effective immunosuppressive drugs like calcineurin inhibitors and mycophenolic acid, which have significantly reduced acute rejection. However, long-term graft survival remains a challenge due to drug toxicity and side effects, prompting ongoing research into safer and more effective alternatives like belatacept and mTOR inhibitors.
The success of kidney transplantation also depends on refining immunosuppressive strategies to balance efficacy with safety. Prolonged use of calcineurin inhibitors can cause nephrotoxicity and fibrosis, while steroids are linked to cardiovascular risks. Therefore, current efforts focus on minimizing these drugs through CnI- and steroid-sparing regimens and developing biomarkers to predict long-term graft outcomes. This evolving approach aims to sustain graft health, reduce complications, and further enhance the life-saving potential of kidney transplantation.
T-Lymphocyte Activation and Site of Action of Immunosuppressive Drugs
To understand the mechanism of action of current immunosuppressive medications, it is crucial to understand the T-lymphocyte (T-cell) activation cascade (Figure 1). T-cells are pivotal for the immune system and are therefore responsible for transplant organ rejection.
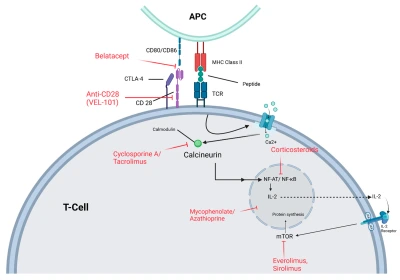
Figure 1. T-cell Activation: T-cell activation begins when the TCR binds to an antigen presented by an APC via MHC class II.
T-cell activation is tightly regulated by both positive and negative signals to maintain immune balance and prevent transplant rejection. It requires two key signals: antigen recognition and co-stimulation. The first occurs when antigen-presenting cells display donor antigens via MHC molecules to T-cells, initiating a weak activation. The second, a co-stimulatory signal through CD28-CD80/86 binding, ensures full activation; without it, T-cells become unresponsive (anergic).
Once activated, T-cells trigger calcium-dependent signaling that activates calcineurin, leading to NFAT-mediated transcription of interleukin-2 (IL-2), which drives T-cell growth and proliferation. IL-2 then binds to its receptor (CD25), activating the mTOR pathway that supports cell cycle progression. Immunosuppressive drugs act by targeting different stages of this activation process, thereby preventing graft rejection.
Current Maintenance Immunosuppression Regimens
Anti-metabolite and CnI-based regimens
Mycophenolic acid and tacrolimus form the foundation of modern immunosuppression. The ELITE Symphony trial showed that low-dose tacrolimus with mycophenolate and steroids provided the best graft survival and lowest rejection rates. Studies suggest maintaining tacrolimus trough levels around 6–8 ng/mL to balance efficacy and toxicity. Mycophenolic acid remains the preferred antimetabolite, showing reduced rejection and graft loss compared to azathioprine. Fixed dosing is commonly used, with adjustments based on side effects rather than drug levels.
Early steroid withdrawal regimens
Early steroid withdrawal (ESW) reduces metabolic side effects like diabetes and weight gain, and is mainly used in low-risk kidney transplant recipients. Trials show comparable survival outcomes between ESW and chronic steroid use, though mild acute rejection is slightly higher with ESW. Long-term data indicate better graft and patient survival, especially with lymphocyte-depleting induction. However, ESW carries higher rejection risks when combined with basiliximab or in re-transplant cases, so careful patient selection is crucial.
mTOR inhibitor-based regimens
mTOR inhibitors (sirolimus, everolimus) can reduce CnI exposure but are linked to higher acute rejection rates. Trials like ZEUS and ELEVATE showed improved kidney function but more side effects and treatment discontinuation in mTOR groups. Combining mTOR inhibitors with reduced-dose CnI provides similar efficacy and fewer viral infections, though proteinuria and adverse effects remain concerns. They are best suited for low-risk patients with GFR > 40 mL/min/1.73 m².
Belatacept-based regimens
Belatacept, a CTLA-4 fusion protein, blocks T-cell co-stimulation and offers better long-term kidney function than CnI-based therapy. Trials like BENEFIT and BEST reported higher early rejection rates but improved graft survival over time. Using short-term tacrolimus with belatacept reduces rejection while maintaining renal benefits. Conversion studies show better kidney function and lower DSA formation after switching from CnI to belatacept, though monitoring for early rejection remains essential.
Practical Considerations
Mycophenolic acid (1000 mg MMF or 720 mg sodium twice daily) with tacrolimus remains standard. Mycophenolate sodium may cause fewer GI side effects, but both can affect growth and appetite. If poorly tolerated or during pregnancy, azathioprine (1–2 mg/kg daily) is preferred. Before starting azathioprine, check TPMT enzyme activity and avoid allopurinol due to risk of cytopenia from drug interaction.
Tacrolimus levels start higher post-transplant and taper to ≥6 ng/mL. Extended-release LCP-tacro helps reduce tremors. When combined with mTOR inhibitors, tacrolimus (4–6 ng/mL) and mTOR-i (3–8 ng/mL) levels must be carefully managed to avoid kidney toxicity. Cyclosporine can replace tacrolimus if not tolerated, with trough targets of 150–250 ng/mL initially and 100–150 ng/mL later. Tacrolimus often causes diabetes and hair thinning, while cyclosporine leads to hirsutism and gum overgrowth. Both may rarely cause PRES (neurological syndrome).
Prednisone is continued in patients with glomerulonephritis (like IgA nephropathy) and those at high immunologic risk or with autoimmune diseases, as it improves outcomes.
mTOR inhibitors are preferred for patients with prior non-melanoma skin cancer due to reduced recurrence risk. However, they delay wound healing, increase lymphocele risk, and can worsen proteinuria, so they should be avoided in patients with active wounds or proteinuric kidney disease.
Belatacept is used for EBV-seropositive kidney recipients to prevent rejection, as EBV-negative patients face a high risk of PTLD. Its broader use is limited by the need for infusion centers, higher early rejection rates, and lack of clarity on dosing during infections or malignancy.
Future Directions
Over the years, advancements in immunosuppression have revolutionized kidney transplantation from an experimental procedure into the preferred treatment for end-stage kidney disease (ESKD). Despite reducing acute rejection rates, long-term graft survival remains suboptimal, partly due to the side effects of current immunosuppressive therapies. The goal remains achieving immune tolerance with minimal adverse effects. CTLA-4, a key immune regulator, affects regulatory T-cell (Treg) development, and its inhibition can promote rejection. To address this, new targets such as VEL-101, a pegylated anti-CD28 antibody fragment, are under study. VEL-101 blocks T-cell activation while preserving CTLA-4’s immunoregulatory function, showing promise as a self-administered, subcutaneous therapy currently in early-phase human trials.
Developing new immunosuppressants also depends on reliable markers for predicting long-term graft survival. Traditional trials focus on short-term outcomes like acute rejection, GFR, and graft loss, where calcineurin inhibitors (CnIs) show strong efficacy but carry chronic toxicity risks. The iBOX model integrates factors such as GFR, proteinuria, donor-specific antibodies, and histologic findings to predict long-term graft failure. Having received approval from both the European Medicines Agency and the U.S. FDA, iBOX represents a major step forward. Its implementation as a clinical endpoint could transform how future immunosuppressive therapies are assessed, ensuring a better balance between efficacy and long-term graft health.
References
1. Shi, B.; Ying, T.; Chadban, S.J. Survival after kidney transplantation compared with ongoing dialysis for people over 70 years of age: A matched-pair analysis. Am. J. Transplant. 2023, 23, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
2. de Boer, S.E.; Knobbe, T.J.; Kremer, D.; van Munster, B.C.; Nieuwenhuijs-Moeke, G.J.; Pol, R.A.; Bakker, S.J.; Berger, S.P.; Sanders, J.S.F. Kidney Transplantation Improves Health-Related Quality of Life in Older Recipients. Transpl. Int. 2024, 37, 12071. [Google Scholar] [CrossRef]
3. Ryu, J.-H.; Koo, T.Y.; Ro, H.; Cho, J.-H.; Kim, M.-G.; Huh, K.H.; Park, J.B.; Lee, S.; Han, S.; Kim, J.; et al. Better health-related quality of life in kidney transplant patients compared to chronic kidney disease patients with similar renal function. PLoS ONE 2021, 16, e0257981. [Google Scholar] [CrossRef] [PubMed]
4. Starzl, T.E. The early days of transplantation. JAMA 1994, 272, 1705. [Google Scholar] [CrossRef]
5. Jing, L.; Yao, L.; Zhao, M.; Peng, L.-P.; Liu, M. Organ preservation: From the past to the future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef]
6. Colombo, D.; Ammirati, E. Cyclosporine in transplantation—A history of converging timelines. J. Boil. Regul. Homeost. Agents 2011, 25, 493–504. [Google Scholar]
7. Tedesco, D.; Haragsim, L. Cyclosporine: A Review. J. Transplant. 2012, 2012, 230386. [Google Scholar] [CrossRef]
8. Burdmann, E.A.; Andoh, T.F.; Yu, L.; Bennett, W.M. Cyclosporine nephrotoxicity. Semin. Nephrol. 2003, 23, 465–476. [Google Scholar] [CrossRef]
9. Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef]
10. Clarkson, M.R.; Sayegh, M.H. T-cell costimulatory pathways in allograft rejection and tolerance. Transplantation 2005, 80, 555–563. [Google Scholar] [CrossRef]