Introduction
(Article introduction authored by Conquest Editorial Team)
Drugs offer therapeutic benefits but can also cause adverse drug reactions (ADRs), ranging from mild to severe, including disability and death. Clinical trials, pharmacovigilance, and pharmacoepidemiology studies help assess the drug’s risk-benefit ratio. Patients with chronic kidney disease (CKD), often excluded from trials, are particularly prone to ADRs due to factors like older age and polypharmacy.
However, it’s unclear if kidney function directly affects ADR risk. CKD alters medication pharmacodynamics and pharmacokinetics, influencing drug effectiveness and safety. This study hypothesizes that lower kidney function increases the risk of serious ADRs, independent of age and polypharmacy. It uses the CKD-REIN cohort to describe serious ADRs, identify preventable ADRs, and explore the link between kidney function and common serious ADRs.
Methods
The CKD-REIN study is a prospective cohort study conducted across 40 nephrology centers in France. It included patients aged 18 or older with moderate or advanced CKD (eGFR < 60 mL/min/1.73 m2), not on dialysis or with a kidney transplant. Enrolled from July 2013 to March 2016, the study involved 3,033 patients who provided informed consent. Approved by INSERM and registered at ClinicalTrials.gov (NCT03381950), the study follows STROBE guidelines for reporting.
Study Design and Participants
CKD-REIN is a prospective cohort study in 40 nephrology centers in France, involving patients aged 18 or older with moderate or advanced CKD (eGFR < 60 mL/min/1.73 m2), not on dialysis or with a kidney transplant.
From July 2013 to March 2016, 3,033 patients provided informed consent. The study is approved by INSERM and registered at ClinicalTrials.gov (NCT03381950), following STROBE guidelines.
Data were collected at baseline and annually by trained clinical research associates, including sociodemographic characteristics, medical history, medication adherence (Girerd score), and lab measurements (hemoglobin, creatinine, albumin, and urine ratios).
Medication use was tracked from 3 months before enrollment. Kidney replacement therapy (KRT) initiation and deaths were recorded from patient reports, medical records, or national registries.
Identification and Adjudication of Adverse Drug Reactions
According to the WHO, an adverse drug reaction (ADR) is a harmful or unpleasant response to a medicinal product that may necessitate changes in treatment or withdrawal of the drug. Serious ADRs include death, life-threatening situations, hospitalization, disability, or important medical events.
In CKD patients, drug-induced acute kidney injury (AKI) and bleeding are high-risk events. AKI is defined by specific increases in serum creatinine, while serious bleeding is characterized by fatal or critical symptomatic hemorrhage or anemia.
For the study, ADRs were tracked over 5 years using electronic forms. Data sources included medical records, patient interviews, and hospital reports. Pharmacists reviewed ADRs to assess causality, seriousness, and management actions. Serious ADRs were further evaluated by a committee of expert
pharmacologists to determine causality, consequences, and preventability.
Assessment of the Causes and Preventability of Serious Adverse Drug Reactions
The pharmacovigilance committee used the Bégaud method for assessing causality, evaluating each drug independently for its role in the ADR. The Naranjo algorithm was also employed to confirm the causal relationship. ADRs were included only if deemed at least “possible” by both methods.
Preventability was rated on a 7-item scale, categorizing ADRs as preventable, potentially preventable, not assessable, or not preventable. This assessment considered prescription compliance, risk factors, and medication errors. If compliance or need was uncertain, the ADR was rated as “not assessable.” For preventable cases, factors such as prescription appropriateness and self-medication were reviewed.
Statistical Analyses
Baseline characteristics were summarized for all participants, using mean ± SD, median [IQR], or number (percentage). Crude incidence rates of serious ADRs were calculated per 100 person-years for the overall population and by ADR type. Incidence rates were compared using quasi-Poisson model deviance tests based on baseline eGFR levels (≥30 vs <30 mL/min/1.73 m2).
Cause-specific Cox proportional hazard models assessed the link between baseline eGFR and the first serious ADR, adjusted for baseline characteristics. Data were censored at death, KRT initiation, or last follow-up. Models included variables such as age, sex, serum albumin, diabetes, and medication adherence. Further adjustments were made based on ADR type. Restricted cubic splines were used to explore the relationship between eGFR and ADR risks. Secondary analyses focused on preventable versus nonpreventable serious ADRs. Missing data were addressed using multivariate imputation by chained equations, and statistical analyses were performed with R software.
Results
Baseline Characteristics: Two-thirds of participants were men. The median age was 69 years, with patients taking a median of 8 medications. High prevalence of polypharmacy and hyperpolypharmacy was observed (81% and 25%, respectively). Hypertension was present in 91% of participants, while 43% had diabetes and 53% had cardiovascular disease.
Characteristics of Adverse Drug Reactions (ADRs): Over a median follow-up of 4.7 years, 1,672 ADRs were reported in 32% of participants, with 488 classified as serious. Nonserious ADRs were most commonly gastrointestinal, followed by kidney and urinary disorders. Serious ADRs frequently involved kidney and urinary disorders and bleeding events, especially in patients with eGFR < 30 mL/min/1.73 m2.
Patients reported only one-third of total ADRs, with nonserious ADRs more frequently reported (44%) compared to serious ADRs (8%). Antithrombotic agents and renin-angiotensin system inhibitors were the primary drugs responsible for ADRs. Serious ADRs often involved multiple drugs and drug interactions. Drug-related AKI and bleeding events were particularly noted for interactions.
The majority of drugs responsible for serious ADRs had been used chronically. Discontinuation of the drug was the most common response to serious ADRs, followed by dose adjustment and no change.
Short-term Consequences and Preventability of Serious Adverse Drug Reactions Among the 488 serious ADRs, 21 were medically significant but did not involve hospitalization. Of the 467 serious ADRs associated with hospitalization, the most common outcome was hospitalization or prolonged hospitalization (365 cases). Life-threatening ADRs occurred in 22 cases, and 32 ADRs were linked to the death of 30 patients (with 2 patients having 2 ADRs each).
Over 27% of serious ADRs were preventable (54 cases) or potentially preventable (78 cases), primarily involving drug-related AKI and bleeding. Preventability was higher for ADRs causing hospitalization (32%) compared to those occurring during hospitalization (18%). Common preventability issues included non-compliance with product guidelines and medication errors by patients. Nearly a third of serious ADRs, especially drug-induced AKIs, occurred during hospitalization. Most patients (73%) recovered without sequelae after a serious ADR.
Kidney Function and Serious Adverse Drug Reactions
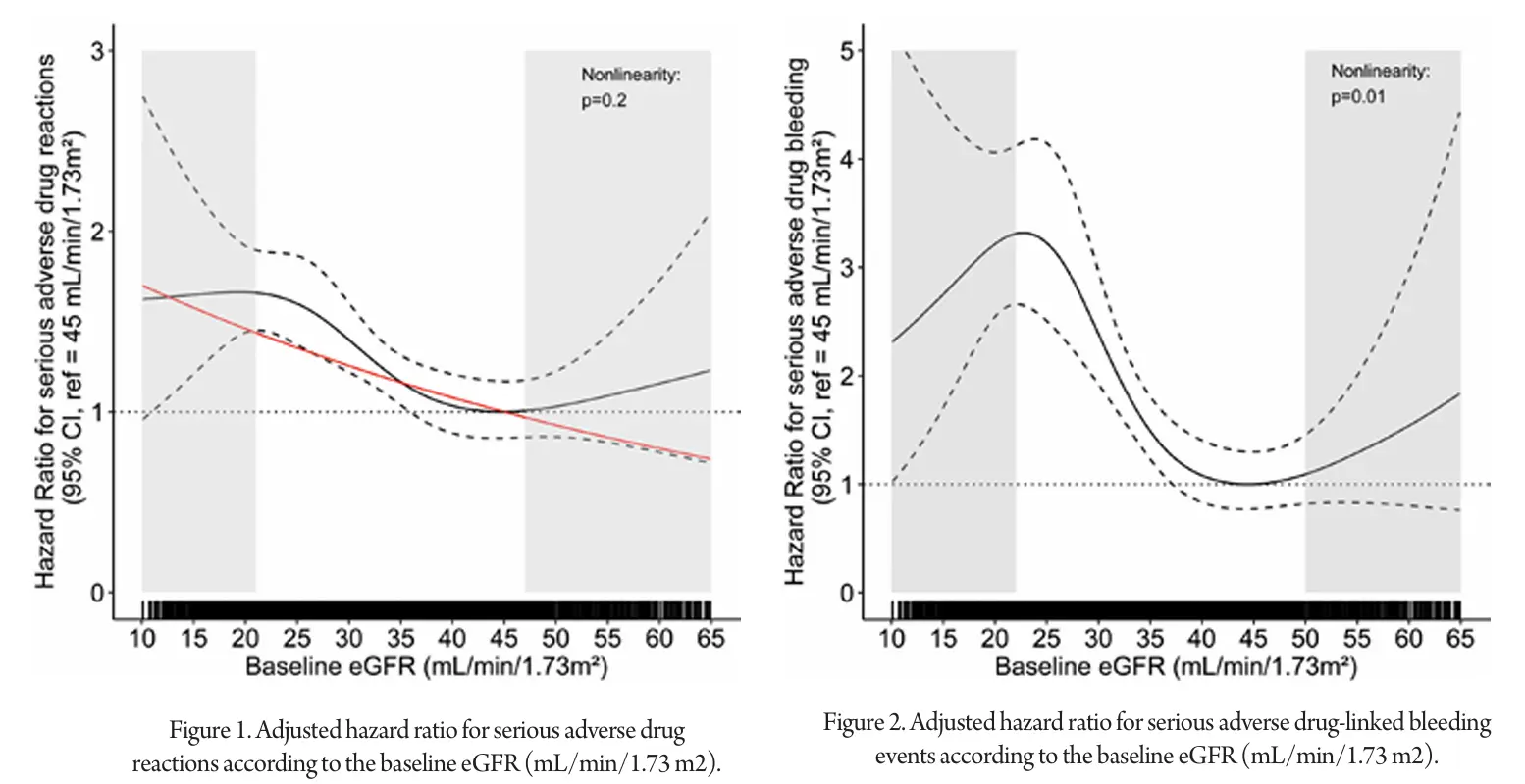
The incidence rate of serious ADRs was significantly higher in patients with a baseline eGFR < 30 mL/min/1.73 m2 compared to those with eGFR ≥ 30 mL/min/1.73 m2 (P < 0.001). Specifically, serious ADRs related to antithrombotics were more common in patients with eGFR < 30 mL/min/1.73 m2 (P < 0.001), while the incidence of serious ADRs linked to RAS inhibitors did not significantly differ by eGFR (P = 0.4). Graphical analysis using restricted cubic splines revealed a linear relationship between eGFR and the hazard ratio for serious ADRs within the eGFR range of 25 to 38 mL/min/1.73 m2. Within this range, each 1 mL/min/1.73 m2 decrease in eGFR was associated with a 2.8% increase in the risk of serious ADRs. Outside this range, the trend was less clear.
For serious ADRs, the risk of serious drug-induced AKI increased by 2.2% for each 1 mL/min/1.73 m2 decrease in baseline eGFR, with a linear relationship confirmed (P = 0.8). The relationship between serious bleeding ADRs and baseline eGFR was nonlinear (P = 0.01). However, within the eGFR range of 25 to 38 mL/min/1.73 m2, a linear relationship was observed, with each 1 mL/min/1.73 m2 decrease in eGFR increasing the risk of serious bleeding ADR by 8%.
Discussion
In a large cohort of nondialysis CKD patients, we extensively reviewed ADRs over a 5-year period using validated tools. ADRs were common, affecting 32% of patients, with 12% experiencing serious ADRs. Many serious ADRs were preventable, often due to noncompliance with prescription guidelines. The most frequent serious ADRs were drug-induced AKI and bleeding, which often required hospitalization and sometimes led to death. Lower eGFR was a significant risk factor for serious ADRs, including bleeding and drug-linked AKI.
The study revealed high incidence rates for serious ADRs, primarily driven by AKI and bleeding. The elevated ADR rates in CKD patients underscore the need for careful management, as these ADRs can lead to severe outcomes and increased healthcare costs. Over a quarter of serious ADRs were preventable, typically due to inappropriate prescriptions or self-medication. Improving patient education about drug safety and ADR symptoms is crucial, as only 33% of ADRs were reported by patients.
The study also highlights the increased risk of serious ADRs with lower eGFR, especially concerning antithrombotic agents and RAS inhibitors. Effective management and patient education are essential to mitigate these risks. Despite the study’s strengths, including comprehensive data and long follow-up, some ADRs might have been missed. Future research should focus on enhancing ADR reporting and management strategies in CKD patients to improve safety and outcomes.
References
1. S.M. Laville, V. Gras-Champel, J. Moragny, et al. Adverse drug reactions in patients with CKD Clin J Am Soc Nephrol, 15 (8) (2020), pp. 1090-1102, 10.2215/CJN.01030120
2. J.S. Ginsberg, M. Zhan, C.J. Diamantidis, C. Woods, J. Chen, J.C. Fink Patient-reported and actionable safety events in CKD J Am Soc Nephrol, 25 (7) (2014), p. 1564, 10.1681/ASN.2013090921
3. F.S. Sharif-Askari, S.A.S. Sulaiman, N.S. Sharif-Askari, A.A.S. Hussain Development of an adverse drug reaction risk assessment score among hospitalized patients with chronic kidney disease PLOS One, 9 (4) (2014), Article e95991, 10.1371/journal.pone.0095991
4. R. Pecoits-Filho, D. Fliser, C. Tu, et al. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care J Clin Hypertens, 21 (7) (2019), pp. 991-1001, 10.1111/jch.13563
5. A. Al Hamid, M. Ghaleb, H. Aljadhey, Z. Aslanpour A systematic review of hospitalization resulting from medicine-related problems in adult patients Br J Clin Pharmacol, 78 (2) (2014), pp. 202-217, 10.1111/bcp.12293
6. C. André, G. Choukroun, Y. Bennis, et al. Potential interactions between uraemic toxins and drugs: an application in kidney transplant recipients treated with calcineurin inhibitors Nephrol Dial Transplant, 37 (11) (2022), pp. 2284-2292, 10.1093/ndt/gfab114
7. Cunha RS da, C.A.B. Azevedo, C.A. Falconi, et al. The interplay between uremic toxins and albumin, membrane transporters and drug interaction Toxins, 14 (3) (2022), p. 177,
8. S.K. Nigam, W. Wu, K.T. Bush, M.P. Hoenig, R.C. Blantz, V. Bhatnagar Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters Clin J Am Soc Nephrol, 10 (11) (2015), p. 2039, 10.2215/CJN.02440314
9. B. Stengel, C. Combe, C. Jacquelinet, et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study Nephrol Dial Transplant, 29 (8) (2014), pp. 1500-1507, 10.1093/ndt/gft388
10. B. Stengel, M. Metzger, C. Combe, et al. Risk profile, quality of life and care of patients with moderate and advanced CKD. The French Chronic Kidney Disease–Renal Epidemiology and Information Network (CKD-REIN) Cohort Study Nephrol Dial Transplant, 34 (2) (2019), pp. 277-286, 10.1093/ndt/gfy058
11. Elm E von, D.G. Altman, M. Egger, S.J. Pocock, P.C. Gøtzsche, J.P. Vandenbroucke The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies Lancet, 370 (9596) (2007), pp. 1453-1457, 10.1016/S0140-6736(07)61602-X
12. X. Girerd, A. Radauceanu, J.M. Achard, et al. Evaluation of patient compliance among hypertensive patients treated by specialists [in French] Arch Mal Coeur Vaiss, 94 (8) (2001), pp. 839-842
13. A.S. Levey, L.A. Stevens, C.H. Schmid, et al. A new equation to estimate glomerular filtration rate Ann Intern Med, 150 (9) (2009), pp. 604-612,10.7326/0003-4819-150-9-200905050-00006
14. World Health Organization Collaborating Centre for Drug Statistics Methodology, Oslo Guidelines for ATC Classification and DDD Assignment 2016. WHO https://web.archive.org/web/20161019104836/http://www.whocc.no/filearchive/publications/2016_guidelines_web.pdf
15. C. Couchoud, B. Stengel, P. Landais, et al. The Renal Epidemiology and Information Network (REIN): a new registry for end-stage renal disease in France