Abstract
Although rates of organ donation and solid organ transplantation have been increasing over the last few decades, demand for organs still greatly exceeds supply. Several strategies have been utilized to increase organ supply, including utilization of high-risk (e.g. HCV antibody-positive) donors. In this context, organs from HCV antibody-positive donors have been used in recipients with chronic HCV since the early 1990s.
Recently, transplantation of HCV-viraemic organs into HCV-naïve recipients has garnered significant interest, owing to the development of safe and highly effective direct-acting antivirals and increased experience of treating HCV in the post-transplant setting. Preliminary studies based largely in the US have shown excellent outcomes in kidney, liver, heart, and lung transplantation. This practice has the potential to significantly increase transplantation rates and decrease waitlist mortality; however, intentionally transmitting an infectious disease to recipients has important practical and ethical implications.
Introduction
Since the early 1990s, HCV antibody-positive (anti-HCV-positive) donor grafts have been used in recipients with chronic HCV, leading to comparable graft and patient outcomes for kidney and liver transplantation. However, using these organs in anti-HCV-negative recipients prior to direct-acting antiviral (DAA) therapy led to high rates of HCV transmission and decreased patient and graft survival.
Over the last decade, the development of highly effectively DAA agents has allowed for the safe and successful treatment of HCV, shrinking the number of recipients with chronic HCV. The high level of safety and efficacy of modern DAA agents in all genotypes, transplant recipients, and patients with renal failure, combined with the increasing availability of anti-HCV-positive donors has led several US transplant centres to pursue transplantation of HCV-viraemic organs into nonviraemic recipients using various strategies for HCV prevention and/or treatment.
HCV-viraemic donors to nonviraemic recipients
Liver
Recent studies prospectively followed 80 HCV-nonviraemic recipients who received anti-HCV-positive nonviraemic livers. HCV viraemia was detected post-transplant in 9 recipients (11.5%), and 7 received DAA therapy, of whom 6 achieved SVR12 . A total of 16 HCV-nonviraemic liver transplant recipients who received viraemic livers with detectable viraemia post-transplant in 94% of recipients. All patients were treated with G/P and 13/16 achieved SVR12 with 100% patient and graft survival at median follow-up of 8 to 11 months. (Fig 1)
Fig 1. A study on HCV-nonviraemic liver transplant recipients who received viraemic livers

Kidney
The first prospective trial, THINKER in 2017 followed by THINKER-2, included 20 HCV-nonviraemic recipients who all received viraemic kidneys with genotype 1, requiring a rapid pre-transplant donor genotyping strategy. All patients had detectable HCV viraemia post-transplant and were subsequently started on elbasvir/grazoprevir (EBR/GZR) for 12 weeks or EBR/GZR/ribavirin (RBV) for 16 weeks if there were NS5A resistance-associated substitutions. All patients had sustained virological response at 12 weeks post-treatment (SVR12) and renal allograft function at 6 months was beater than matched controls, based on the Organ Procurement and Transplantation Network (OPTN) registry.
Heart
Thre first prospective study using HCV-viraemic hearts included 11 nonviraemic recipients who were then followed by NAT post-transplant. Nine out of 11 (85%) developed detectable HCV viraemia post-transplant and were started on SOF/ledipasvir (LDV) for 12 weeks (genotype 1) or SOF/VEL for 12–24 weeks (genotype 3), as outpatients, at a median 33 days post-transplant – 100% achieved SVR12.
In another study, a total of 165 HCV-nonviraemic recipients received viraemic hearts followed by surveillance NAT and treatment with various DAA regimens if positive. 159/165 (96%) had detectable HCV viraemia post-transplant with SVR12 in 118 patients (4 patients died without viraemia prior to finishing therapy, 37 patients were in various stages of treatment at publication). Reported 1-year survival rates were between 91–95%. (Fig 2)
Fig 2. A study on HCV-nonviraemic heart transplant recipients who received viraemic hearts

Lung
The first report of HCV-viraemicc donors in lung transplantation reviewed 5 cases where HCV-nonviraemic recipients were rapidly deteriorating and accepted viraemic lungs. All patients had detectable HCV viraemia post-transplant, as well as SVR12 with SOF/LDV or SOF/VEL for 12 weeks. Median time to initiation of therapy was 28 days with no interruptions in therapy or adverse events related to HCV or DAA therapy.
The DONATE-HCV trial also included 36 HCV-nonviraemic recipients who received viraemic lungs followed by SOF/VEL immediately post-transplant for 4 weeks. 34/36 (94%) had detectable viraemia post-transplant and 28/28 had achieved SVR12 at 6 months of follow-up. (Fig 3)
Fig 3. A study on HCV-nonviraemic lung transplant recipients who received viraemic lungs

Special considerations
While the preliminary data regarding HCV-viraemic organs is promising, there are still many unanswered questions, such as the risks and benefits of this strategy (Table 1) and any special generalisable or organ-special considerations (Fig 4).
Table 1. Benefits and risks of transplanting HCV-infected organs into HCV-uninfected recipients.
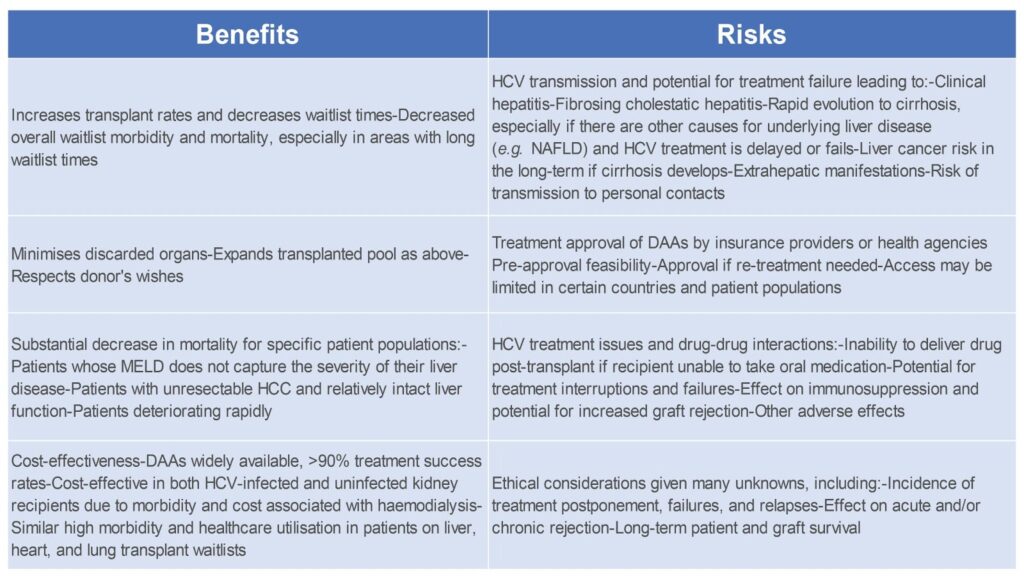
Fig 4. Special considerations for hepatitis C virus infection in solid organ transplantation.

Currently, anti-HCV-positive and viraemic donors are evaluated using the standard quality measures accepted for each organ. Regarding HCV NAT-positive kidney donors, several of the pilot trials used a cut-off for kidney donor profile index (KDPI), a measure of organ quality, of 85% and age of 55 years. Similarly, most of the data regarding acceptable degree of fibrosis in donor livers comes from the pre-DAA era; however, there appears to be no difference in post-transplant fibrosis progression rates between grade 0 and grade 1 or 2 fibrosis, nor between steatosis score 0 and >0.
Donor HCV genotype clearly impacts treatment regimens and resistance patterns, and it has been shown that people who use injection drugs have higher rates of genotype 1a and 3, which are associated with more RASs. However, DAA regimens have been developed that overcome most RASs, and most preliminary studies included multiple genotypes. One of the biggest questions relating to the utilisation of HCV-viraemic organs is recipient selection.
A cost-effective analysis suggested that postponing HCV treatment and accepting an HCV-viraemic kidney was preferable to pre-transplant HCV treatment and receiving an HCV-nonviraemic kidney unless the additional wait time for a nonviraemic kidney was less than 161 days.
For patients with decompensated cirrhosis and chronic HCV, the decision to treat their HCV pre- or post-transplant is determined by the degree of liver dysfunction (Fig. 5), as there are some patients who may improve their liver function enough that they may no longer require liver transplantation.
Fig 5. Special considerations for hepatitis C virus infection in solid organ transplantation.

Conclusion
Utilisation of anti-HCV-positive viraemic and nonviraemic organs decreases waitlist times by minimising discarded organs and significantly expanding the available donor pool. The practice has the potential to significantly decrease overall waitlist morbidity and mortality, especially in areas with long waiting list times.
In the DAA era, this strategy has been shown to be safe and cost-effective in HCV-viraemic recipients, and, more recently, there is a rapidly growing literature that preliminarily suggests this may be true in HCV-nonviraemic recipients as well. However, there is still limited data on non-clinical trial experience with this strategy and a lack of long-term outcome data.
Lastly, fundamental to this process is the ability to secure DAA therapy prior to transplant and ensure that patients are fully informed regarding the associated risks, including the potential for HCV treatment failure and its consequences.
References:
- Organ procurement and transplantation network. (Available at:)https://optn.transplant.hrsa.gov
- Scholz N. Organ donation and transplantation. Facts, figures and European Union action [Internet].(Available at:) https://www.europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
- Morales J. M.Campistol J.M.Domínguez-Gil B.Andrés A.Esforzado N.Oppenheimer F.et al. Long-term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients.
- Scalea J.R., Barth R.N., Munivenkatappa R., Philosophe B., Cooper M., Whitlow V., et al. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts.
- Northup P.G., Argo C.K., Nguyen D.T., McBride M.A., Kumer S.C., Schmitt T.M., et al. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int. 2010; 23: 1038-1044
- Ballarin R., Cucchetti A., Spaggiari M., Montalti R., Di Benedetto F. ,Nadalin S., et al. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011; 91: 1265-1272
- Gasink L.B.,Blumberg E.A., Localio A.R.,Desai S.S., Israni A.K., Lautenbach E. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. J Am Med Assoc. 2006; 296: 1843-1850
- Flohr T.R. Bonatti H. Hranjec T. Keith D.S. Lobo P.I. Kumer S.C. et al. Elderly recipients of hepatitis C positive renal allografts can quickly develop liver disease. J Surg Res. 2012; 176: 629-638
- Englum B.R. Ganapathi A.M. Speicher P.J. Gulack B.C. Snyder L.D. Duane Davis R. et al. Impact of donor and recipient hepatitis C status in lung transplantation. J Heart Lung Transplant. 2016; 35: 228-235
- Gupta G. Kang L. Yu J.W. Limkemann A.J. Garcia V. Bandyopadhyay D. et al. Long-term outcomes and transmission rates in hepatitis C virus-positive donor to hepatitis C virus-negative kidney transplant recipients: analysis of United States national data. Clin Transplant. 2017; 31: 1-9
- Falade-Nwulia O. Suarez-Cuervo C. Nelson D.R. Fried M.W. Segal J.B. Sulkowski M.S. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017; 166: 637-648
- Goldberg D.S. Blumberg E. Mccauley M. Abt P. Levine M. Improving organ utilization to help overcome the tragedies of the opioid epidemic. Am J Transplant. 2016; 16: 2836-2841
- Durand C.M. Bowring M.G. Thomas A.G. Kucirka L.M. Massie A.B. Cameron A. et al. The drug overdose epidemic and deceased-donor transplantation in the United States: a national registry study. Ann Intern Med. 2018; 168: 702-711
- Mansour M. Hill L. Kerr J. Safety and effectiveness of direct acting antivirals for treatment of hepatitis C virus in patients with solid organ transplantation.
- Puoti M. Foster G.R. Wang S. Mutimer D. Gane E. Moreno C. et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1–6 patients without cirrhosis. J Hepatol. 2018; 69: 293-300
- Roth D. Nelson D.R. Bruchfeld A. Liapakis A. Silva M. Monsour H. et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015; 386: 1537-1545
- Goldberg D.S. Abt P.L. Blumberg E.A. Van Deerlin V.M. Levine M. Reddy K.R. et al. Trial of transplantation of HCV-infected kidneys into uninfected recipients.N Engl J Med. 2017; 376: 2394-2395
- Abdelbasit A. Hirji A. Halloran K. Weinkauf J. Kapasi A. Lien D. et al. Lung transplantation from hepatitis C viremic donors to uninfected recipients. Am J Respir Crit Care Med. 2018; 197: 1492-1496
- Durand C.M. Bowring M.G. Brown D.M. Chattergoon M.A. Massaccesi G. Bair N. et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients an open-label nonrandomized trial.
- Kwong A.J. Wall A. Melcher M. Wang U. Ahmed A. Subramanian A. et al. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant. 2018; 19: 1380-1387
- Reese P.P. Abt P.L. Blumberg E.A. Van Deerlin V.M. Bloom R.D. Potluri V.S. et al. Twelve-month outcomes after transplant of hepatitis C–infected kidneys into uninfected recipients a single-group trial. Ann Intern Med. 2018; 169: 273-281
- Schlendorf K.H. Zalawadiya S. Shah A.S. Wigger M. Chung C.Y. Smith S. et al. Early outcomes using hepatitis C–positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018; 37: 763-769
- Aslam S. Yumul I. Mariski M. Pretorius V. Adler E. Outcomes of heart transplantation from hepatitis C virus–positive donors. J Hear Lung Transpl [Internet]. 2019; 38: 1259-1267
- Bethea E. Gaj K. Gustafson J.L. Axtell A. Lebeis T. Schoenike M. et al. Pre-emptive pangenotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative heart transplantation: an open-label study. Lancet Gastroenterol Hepatol. 2019; 4: 771-780
- Gupta G. Yakubu I. Bhati C.S. Zhang Y. Kang L. Patterson J.A. et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Am J Transplant. 2019; 20: 739-751
- McLean R.C. Reese P.P. Acker M. Atluri P. Bermudez C. Goldberg L.R. et al. Transplanting hepatitis C virus–infected hearts into uninfected recipients: a single-arm trial. Am J Transplant. 2019; 19: 2533-2542
- Morris K.L. Adlam J.P. Padanilam M. Patel A. Garcia-Cortes R. Chaudhry S.P. et al. Hepatitis C donor viremic cardiac transplantation: a practical approach. Clin Transplant. 2019; 34: 1-4
- Schlendorf K.H. Zalawadiya S. Shah A.S. Perri R. Wigger M. Brinkley D.M. et al. Expanding heart transplant in the era of direct-acting antiviral therapy for hepatitis C. JAMA Cardiol. 2019; 5: 167-174
- Woolley A.E. Singh S.K. Goldberg H.J. Mallidi H.R. Givertz M.M. Mehra M.R. et al. Heart and lung transplants from HCV infected donors to uninfected recipients. N Engl J Med. 2019; 380: 1606-1617
- Bethea E. Arvind A. Gustafson J. Andersson K. Pratt D. Bhan I. et al. Immediate administration of antiviral therapy after transplantation of hepatitis C-infected livers into uninfected recipients: implications for therapeutic planning.





