Introduction
Inflammatory arthritis encompasses a range of immune-mediated joint disorders such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), which lead to chronic joint damage and systemic complications. Despite advancements in conventional and biologic DMARDs, many patients experience inadequate responses due to disease complexity and comorbidities. This review explores the evolving treatment landscape with a focus on rituximab, apremilast, and upadacitinib—three agents that reflect distinct immunomodulatory strategies. By examining their clinical efficacy and safety, especially in difficult-to-treat RA, the review highlights the importance of personalized and targeted therapeutic approaches.
Biosimilar Disease-Modifying Antirheumatic Drugs (bsDMARDs)
BsDMARDs (biosimilar DMARDs) provide cost-effective alternatives to original biologics, improving access to targeted arthritis therapies. Though structurally complex, biosimilars closely match reference drugs in efficacy and safety. Clinical trials are essential to confirm their pharmacokinetics, safety, and immunogenicity, as slight variability can impact their performance.
Anti-CD20–Mediated B-Cell Depletion Agent—Rituximab
Rituximab (RTX), a monoclonal antibody targeting CD20 on B cells, is widely used in the treatment of B-cell malignancies like non-Hodgkin lymphoma and CLL, as well as autoimmune diseases such as rheumatoid arthritis (RA). Its mechanism of action involves depleting B cells through multiple immune pathways, including CDC, ADCC, and ADCP. In RA, RTX is effective for patients who fail to respond to TNF inhibitors and can be safely combined with methotrexate. (Fig 1)It also shows promise in treating RA-related complications such as ILD, pericarditis, and autoimmune cytopenias. While infusion reactions are common—especially during the first dose—serious adverse events like hypogammaglobulinemia, late-onset neutropenia, and HBV reactivation remain rare but significant.
RTX biosimilars like CT-P10 and GP2013 have made treatment more accessible by offering cost-effective options with comparable efficacy and safety to the original drug. Their approval has expanded therapeutic options in both oncology and rheumatology, especially in low-resource settings. Despite some challenges—such as defining optimal dosing, managing resistance, and monitoring for rare side effects—RTX continues to play a key role in managing B-cell-driven diseases. Advances in biomarker-based treatment strategies and further clinical research will help refine its use for better personalized care.
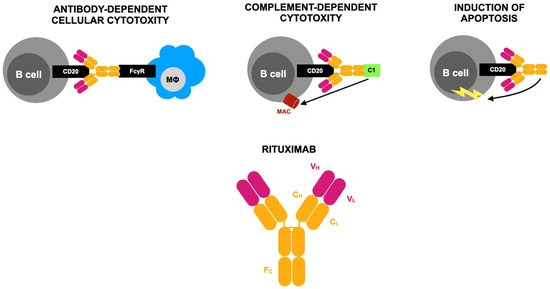
Figure 1.
Targeted Synthetic Disease-Modifying Antirheumatic Drugs (tsDMARDS)
Recent studies have highlighted the importance of signaling pathway dysfunction—especially involving PDE4 and JAK/STAT—in the development of psoriatic arthritis (PsA). Targeted synthetic DMARDs (tsDMARDs), including PDE4 inhibitors like apremilast and JAK inhibitors such as tofacitinib, baricitinib, filgotinib, and upadacitinib, offer new oral treatment options. These small-molecule drugs help reduce inflammation by regulating cytokine production and immune responses.
While effective, JAK inhibitors may cause side effects like serious infections, kidney and liver issues, anemia, and blood clots. These tsDMARDs are generally used when patients do not respond to biologics or prefer oral treatment. Though currently used after biologics, research is exploring their earlier use in RA management. They can be used alone or with conventional DMARDs but not in combination with biologic DMARDs.
PDE4 Inhibitor—Apremilast
Apremilast, an oral PDE4 inhibitor, is approved for treating moderate to severe psoriatic arthritis (PsA) and plaque psoriasis. It works by increasing cAMP levels, which suppress pro-inflammatory cytokines (like TNF-α, IL-23) and reduce joint and skin inflammation. (Fig 2) Clinical trials (PALACE, ESTEEM, ACTIVE) and real-world data have shown it improves symptoms like dactylitis, enthesitis, fatigue, and skin lesions, with benefits seen as early as week 2 and sustained for up to five years. It is especially suitable for patients who prefer oral therapy or have comorbidities that limit biologic use, and it typically requires no lab monitoring.
Although less potent than biologics, apremilast has a favorable safety profile, with the most common side effects being mild gastrointestinal issues. It is often used after csDMARD failure or in biologic-naïve patients and may also be beneficial in off-label indications like Behçet’s disease, atopic dermatitis, and hidradenitis suppurativa. However, treatment persistence may be low due to limited efficacy in severe joint symptoms. Despite this, apremilast remains a versatile second- or third-line option, especially for those at high risk of infections or cancer. Further research is needed to enhance its utility, explore biomarkers for response prediction, and improve outcomes in severe or refractory cases.
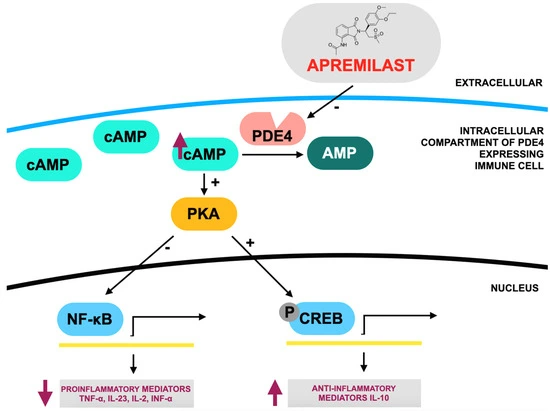
Figure 2.
JAK1 Inhibitor—Upadacitinib
Upadacitinib is a selective JAK1 inhibitor approved for the treatment of multiple chronic inflammatory diseases, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), ulcerative colitis, Crohn’s disease, and atopic dermatitis (AD). It works by disrupting cytokine signaling through the JAK-STAT pathway, reducing inflammation and improving symptoms like joint pain, skin lesions, and fatigue. Clinical trials such as the SELECT and U-EXCEL programs have demonstrated its superior efficacy over placebo and even certain biologics like adalimumab and dupilumab in RA, PsA, and AD.
Upadacitinib is especially beneficial for patients who do not respond to methotrexate or biologic DMARDs. It has shown high remission rates, early symptom control, and sustained improvements in real-world data. However, safety concerns—including risk of serious infections, cardiovascular events, and blood clots—require careful monitoring, especially in older adults or those with comorbidities. Despite these risks, upadacitinib offers a potent oral option, either as monotherapy or in combination, and continues to gain traction as a valuable therapy for difficult-to-treat inflammatory conditions.
Conclusion
Arthritis is a complex autoimmune disease affecting joints and other organs, driven by genetic, environmental, and immune factors. While many treatments exist, some patients still fail to respond adequately, highlighting the need for deeper insights into the disease’s molecular mechanisms and more personalized therapies.
New-generation DMARDs like rituximab, apremilast, and upadacitinib have expanded treatment options. Rituximab, a B-cell targeting antibody, is especially useful in refractory RA and B-cell malignancies. Apremilast, a well-tolerated oral PDE4 inhibitor, is ideal for patients with comorbidities or those avoiding injections. Upadacitinib, a JAK1 inhibitor, offers fast symptom relief in moderate-to-severe autoimmune diseases but requires monitoring for cardiovascular risks.
Together, these agents offer diverse, flexible treatment strategies tailored to individual needs. Their unique mechanisms, delivery methods, and safety profiles support integrated, cross-specialty care. Ongoing research aims to refine therapy selection, explore combination regimens, and enhance long-term patient outcomes in the evolving landscape of precision medicine.
References
1. Sharma, A.; Goel, A. Pathogenesis of rheumatoid arthritis and its treatment with anti-inflammatory natural products. Mol. Biol. Rep. 2023, 50, 4687–4706.
2. Khurana, R.; Berney, S.M. Clinical aspects of rheumatoid arthritis. Pathophysiology 2005, 12, 153–165.
3. Brock, J.; Basu, N.; Schlachetzki, J.C.M.; Schett, G.; McInnes, I.B.; Cavanagh, J. Immune mechanisms of depression in rheumatoid arthritis. Nat. Rev. Rheumatol. 2023, 19, 790–804.
4. Ionescu, C.-E.; Popescu, C.C.; Agache, M.; Dinache, G.; Codreanu, C. Depression in Rheumatoid Arthritis: A Narrative Review-Diagnostic Challenges, Pathogenic Mechanisms and Effects. Medicina 2022, 58, 1637.
5. Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376.
6. Neumann, E.; Frommer, K.; Diller, M.; Müller-Ladner, U. Rheumatoid arthritis. Z. Rheumatol. 2018, 77, 769–775.
7. Adawi, M.; Firas, S.; Blum, A. Rheumatoid Arthritis and Atherosclerosis. Isr. Med. Assoc. J. 2019, 21, 460–463.
8. England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036.
9. Gupta, N.; Kanwar, N.; Arora, A.; Khatri, K.; Kanwal, A. The inter-play of rheumatoid arthritis and osteoporosis: Exploring the pathogenesis and pharma-cological approaches. Clin. Rheumatol.
10. Jang, S.; Kwon, E.-J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905.