Introduction
Calcineurin inhibitors (CNIs), primarily tacrolimus, are widely used as standard maintenance immunosuppressants in liver transplant (LT) recipients. However, their long-term use is often associated with significant adverse effects such as nephrotoxicity, chronic kidney disease (CKD), hypertension, diabetes, and infections. As a result, strategies like immunosuppression minimization or switching to alternative agents—such as mycophenolate mofetil monotherapy (MMF-MT)—have gained attention to mitigate these complications while maintaining graft tolerance and patient survival. MMF, through its active form mycophenolic acid, effectively inhibits lymphocyte proliferation but is not without its own side effects, including gastrointestinal discomfort and hematological toxicities.
This retrospective study presents the outcomes of 324 LT patients who were transitioned from CNI-based immunosuppression to MMF-MT due to CNI-related adverse events, with a particular focus on CKD. Over a median MMF-MT duration of 78 months, the switch was associated with significant improvements in renal function—evidenced by increased glomerular filtration rate and decreased serum creatinine—while maintaining a low rate of acute rejection and overall good drug tolerability. With a five-year post-conversion survival rate of 75.3%, these findings support MMF-MT as a viable long-term strategy for managing immunosuppression in LT patients with CNI intolerance or related complications.
Patients and Methods
Population and Study Design (Short Version):
This retrospective single-center cohort study analyzed 324 liver transplant (LT) recipients who were switched from calcineurin inhibitor (CNI)-based immunosuppression to mycophenolate mofetil monotherapy (MMF-MT) between January 1997 and June 2022 due to CNI-related adverse effects such as chronic kidney disease (CKD), hypertension, diabetes, and recurrent biliary infections. Patients included were over 18 years old, had stable graft function, and no acute rejection in the year prior to MMF-MT conversion. Exclusion criteria included liver retransplantation, prior kidney transplant, or hepatocellular carcinoma recurrence. All patients were followed for at least 1.5 years post-conversion.
Data Collection and Analysis:
Baseline and follow-up data included demographic, clinical, and laboratory parameters, MMF-MT duration, adverse effects, rejection episodes, and need for dialysis or kidney transplantation. The study assessed changes in kidney, liver, and hematological parameters from the pre-MMF-MT period to the last follow-up. Data were compared across two eras (1999–2011 and 2012–2023) for outcomes such as survival, rejection, and adverse effects. Statistical analyses included repeated measures, chi-square, Mann–Whitney U test, and Kaplan–Meier survival curves, with p<0.05 considered statistically significant.
Results
Recipient and Donor Characteristics:
Among 1,697 liver transplant recipients treated with CNIs between 1997 and 2022, 324 were converted to MMF monotherapy. The median recipient age was 55 years, and the main indications for LT were alcoholic cirrhosis, HCV-related cirrhosis, and HCC. Most received tacrolimus-based immunosuppression (77.8%). Livers were primarily from donation after brain death (89.2%).
Pre- and Post-MMF-MT Characteristics:
MMF-MT was started a median of 67 months post-LT, with a follow-up of 68 months. Conversion was primarily due to CKD (66.4%), diabetes (18.8%), and hypertension (13%). Acute rejection occurred in 7.4% of patients post-conversion but was managed successfully. MMF-related adverse effects occurred in 14.8%, mostly diarrhea, vomiting, and leukopenia. MMF-MT was withdrawn in 12.9% of patients due to rejection, side effects, or new malignancies.
MMF Dosage and MPA Monitoring:
MPA plasma levels remained stable over time, with no significant differences between the pre-MMF-MT phase and the last outpatient visit. Doses were adjusted individually based on MPA monitoring throughout follow-up.
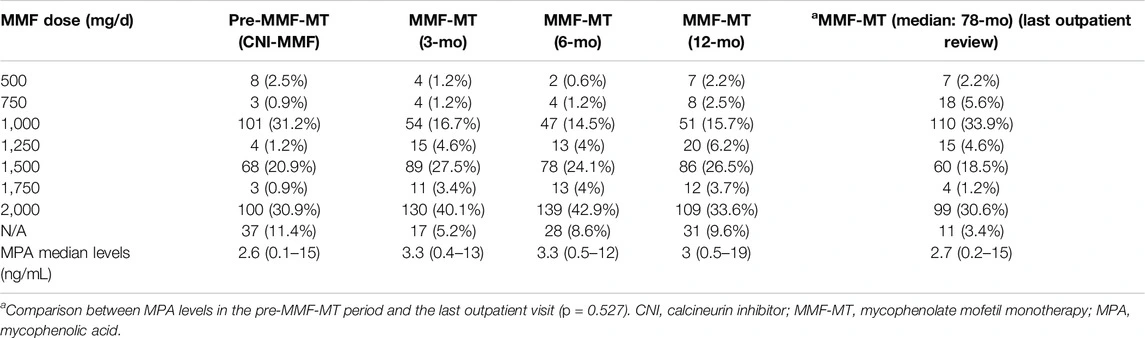
Table 1. Overall daily doses of MMF and monitoring of MPA levels during follow-up.
Comparison Between Pre-MMF-MT and Final Review:
Compared to the pre-MMF-MT phase, patients showed a significant improvement in renal function with increased GFR and decreased creatinine and ALT values. Other variables, including diabetes, hypertension, and hematologic markers, remained stable. Notably, patients switched due to recurrent biliary infections had no further episodes post-conversion.
The overall actuarial patient survival rates at 1, 3, 5, and 10 years after the onset of MMF-MT were 95.7%, 86.5%, 75.3%, and 54.6%, respectively (Figure 3). The actuarial patient survival rate at 1, 3, 5, and 10 years after the onset of MMF-MT were 93.8%, 82.3%, 70.1%, and 51.9%, respectively, in era 1, whereas in the second era patient survival rate was 97.9%, 91.8%, 80.9%, and 64.3%, respectively. (p = 0.089)
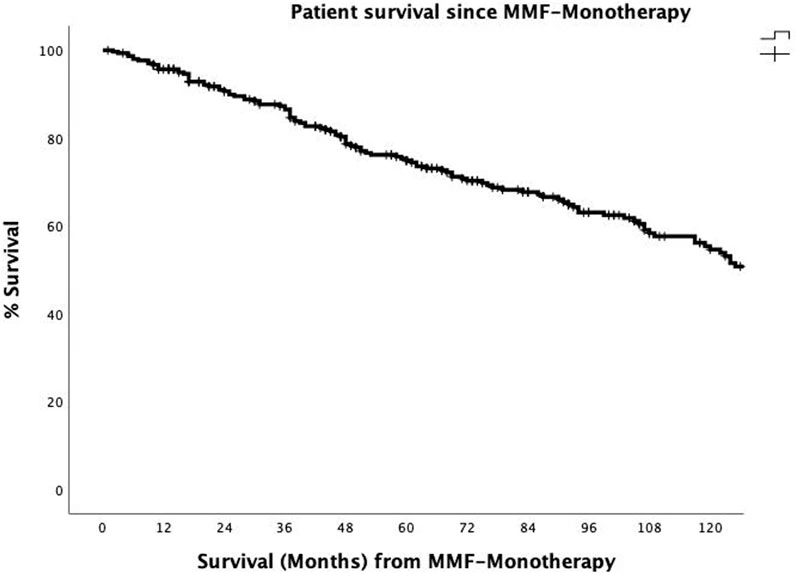
Figure 3. The actuarial patient survival rates after conversion from CNI immunosuppression to MMF-MT (mycophenolate mofetil monotherapy) at 1, 3, 5, and 10 years were 95.7%, 86.5, 75.3%, and 54.6%.
Discussion & Conclusion
CNI Withdrawal and MMF-MT Outcomes:
Prolonged use of CNIs post-liver transplantation is a key risk factor for renal impairment, with complete CNI withdrawal showing better renal outcomes than mere dose reduction. In this study, patients were primarily converted to MMF monotherapy (MMF-MT) due to CKD and other CNI-induced side effects like diabetes and hypertension. A median conversion time of 72 months post-LT and a follow-up of 78 months was observed. Acute rejection occurred in 7.4% of patients post-conversion, with all cases managed successfully using steroids or reintroduction of CNI/mTORi.
Immunosuppression Monitoring and Safety:
MPA plasma level monitoring played a critical role in dose adjustment, minimizing both rejection risk and adverse effects. Most patients tolerated MMF-MT well, with adverse effects occurring in 14.8% and leading to withdrawal in only 2.5%. MMF-MT significantly improved GFR and reduced creatinine and ALT levels over time. No significant changes were seen in diabetes, hypertension, or hematological parameters. The five-year survival rate post-conversion was 75.3%. Despite being a single-center retrospective study, findings support MMF-MT as a safe and effective alternative for LT patients with CNI-related complications.
References
1. Charlton, M, Levitsky, J, Aqel, B, O´Grady, J, Hemibach, J, Rinella, M, et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation (2018) 102:727–43. doi:10.1097/TP.0000000000002147
2. Klupp, J, Pfitzmann, R, Langrehr, JM, and Neuhauss, P. Indications of Mycophenolate Mofetil in Liver Transplantation. Transplantation (2005) 80:S142–6. doi:10.1097/01.tp.0000187133.53916.8f
3. Morard, I, Mentha, G, Spahr, L, Majno, P, Hadengue, A, Huber, O, et al. Long-Term Renal Function After Liver Transplantation Is Related to Calcineurin Inhibitors Blood Levels. Clin Transpl (2006) 20:96–101. doi:10.1111/j.1399-0012.2005.00447.x
4. Ojo, AO. Renal Disease in Recipients of Nonrenal Solid Organ Transplantation. Semin Nephrol (2007) 27:498–507. doi:10.1016/j.semnephrol.2007.03.010
5. Kuo, HT, Sampaio, MS, Ye, X, Reddy, P, Martin, P, and Bunnapradist, S. Risk Factors for New-Onset Diabetes Mellitus in Adult Liver Transplant Recipients, an Analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing Database. Transplantation (2010) 89:1134–40. doi:10.1097/TP.0b013e3181d2fec1
6. Rodríguez-Perálvarez, M, Germani, G, Darius, T, Lerut, J, Tsochatzis, E, and Burroughs, AK. Tacrolimus Through Levels, Rejection and Renal Impairment in Liver Transplantation. A Systematic Review and Meta-Analysis. Am J Transpl (2012) 12:2797–814. doi:10.1111/j.1600-6143.2012.04140.x
7. Carenco, C, Assenat, E, Faure, S, Duny, Y, Danan, G, Bismuth, M, et al. Tacrolimus and the Risk of Solid Cancers After Liver Transplant: A Dose Effect Relationship. Am J Transpl (2015) 15:678–86. doi:10.1111/ajt.13018
8. Karie-Guigues, S, Janus, N, Saliba, F, Dumortier, J, Duvaux, C, Calmus, Y, et al. Long-Term Renal Function in Liver Transplantation Recipients and Impact of Immunosuppressive Regimens [Calcineurin Inhibitors Alone or in Combination With Mycophenolate Mofetil]:The TRY Study. Liver Transpl (2009) 15:1083–91. doi:10.1002/lt.21803
9. Hao, JC, Wang, WT, Yan, LN, Li, B, Wen, TF, Jang, JY, et al. Effect of Low-Dose Tacrolimus With Mycophenolate Mofetil on Renal Function Following Liver Transplantation. World J Gastroenterol (2014) 20:11356–62. doi:10.3748/wjg.v20.i32.11356
10. Duvoux, C, and Pageaux, GP. Immunosuppression in Liver Transplant Recipients With Renal Impairment. J Hepatol (2011) 54:1041–54. doi:10.1016/j.jhep.2010.12.001