Introduction
Calcineurin inhibitors (CNIs), particularly tacrolimus, have long been the cornerstone of maintenance immunosuppression following liver transplantation (LT). While effective in preventing graft rejection, CNIs are frequently associated with significant long-term adverse effects, including nephrotoxicity, chronic kidney disease (CKD), diabetes mellitus, hypertension, infections, and cardiovascular complications. These risks have prompted efforts to modify immunosuppressive regimens to preserve graft function while minimizing harm. One such approach is the substitution of CNIs with mycophenolate mofetil monotherapy (MMF-MT), which has shown promise in improving renal outcomes and reducing CNI-related toxicity, particularly in patients with post-LT CKD or metabolic complications.
In this context, our retrospective study evaluates the outcomes of 324 LT patients who were transitioned from CNI-based immunosuppression to MMF-MT due to CKD and other CNI-related adverse effects. With a median MMF-MT duration of 78 months, the findings highlight significant improvements in renal function, low rejection rates, and favorable long-term tolerance. The most common indications for the switch included CKD, diabetes, hypertension, and recurrent infections, and MMF-MT was largely well-tolerated with minimal discontinuations. As one of the largest single-center studies to assess MMF-MT in LT recipients, this analysis contributes valuable evidence support.
Patients and Methods
This retrospective single-center cohort study analyzed 324 liver transplant (LT) recipients who were converted from calcineurin inhibitor-based immunosuppression to mycophenolate mofetil monotherapy (MMF-MT) between January 1997 and June 2022. These patients were selected from a total of 1,697 individuals who underwent LT during the same period, out of 2,204 total transplants performed at the institution since 1986. The inclusion criteria for MMF-MT conversion were adults over 18 years with stable graft function, no acute rejection in the preceding year, and at least two years of follow-up post-transplant. Exclusion criteria included prior liver retransplantation, renal transplant, or hepatocellular carcinoma recurrence. All patients provided informed consent, and the study adhered to the ethical standards outlined in the Declaration of Helsinki, with approval from the institutional review board.
Baseline patient data and clinical outcomes were retrospectively collected, including demographic information, liver disease severity scores, immunosuppression history, and adverse effects related to CNIs. Post-conversion outcomes such as renal and liver function markers, acute rejection incidence, and MMF-related adverse effects were evaluated at multiple time points. Variables like hypertension, diabetes, and hematologic parameters were compared between the pre- and post-MMF-MT periods using a repeated measures model. The study also stratified patients into two eras (1999–2011 and 2012–2023) for subgroup analysis of rejection rates, adverse events, and survival. Statistical analysis was conducted using SPSS, with significance defined as a p-value <0.05.
Results
Recipient and Donor Characteristics
Out of 1,697 liver transplant (LT) recipients from 1997 to 2022, 324 were included in the study, with most initially on tacrolimus (77.8%) and the rest on cyclosporine. Median recipient age was 55 years, and alcoholic cirrhosis, hepatitis C, and HCC were the most common LT indications.
Pre- and Post-MMF-MT Characteristics
MMF-MT was initiated a median of 67 months post-LT, primarily due to CKD (66.4%), followed by diabetes, hypertension, and biliary infections. Post-conversion rejection occurred in 7.4% of patients, all managed successfully. MMF-related adverse effects were seen in 14.8%, and MMF-MT was discontinued in 12.9%, mainly due to tumors, rejection, or liver/kidney issues.
Dosage of MMF and MPA Monitoring
MMF doses were personalized based on MPA plasma levels. Across the follow-up, MPA levels remained consistent from the pre-MMF-MT phase (2.6 ng/dL) to the final review (2.7 ng/dL), showing no significant variation.
Comparison of Characteristics Between Pre-MMF-MT and Final Review
Long-term follow-up (median 78 months) showed significant improvement in kidney function (GFR increased, creatinine and ALT decreased), while diabetes, hypertension, and liver function remained stable. No recurrent biliary infections were reported post-MMF-MT in affected patients.
Era-Based Comparison
Patients in the first era (1999–2011) had higher pre-LT rates of hypertension and diabetes, while the second era (2012–2023) saw more HCC cases. There were notable differences in reasons for switching to MMF-MT between the two eras.
The overall actuarial patient survival rates at 1, 3, 5, and 10 years after the onset of MMF-MT were 95.7%, 86.5%, 75.3%, and 54.6%, respectively (Figure 1). The actuarial patient survival rate at 1, 3, 5, and 10 years after the onset of MMF-MT were 93.8%, 82.3%, 70.1%, and 51.9%, respectively, in era 1, whereas in the second era patient survival rate was 97.9%, 91.8%, 80.9%, and 64.3%, respectively. (p = 0.089).
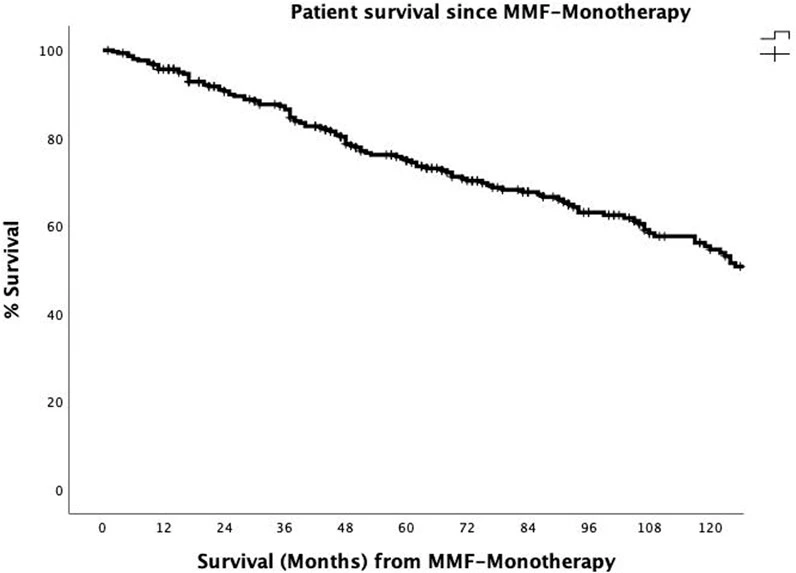
Figure 1. The actuarial patient survival rates after conversion from CNI immunosuppression to MMF-MT (mycophenolate mofetil monotherapy) at 1, 3, 5, and 10 years were 95.7%, 86.5, 75.3%, and 54.6%.
Discussion
Calcineurin inhibitors (CNIs), while effective for immunosuppression after liver transplantation (LT), are associated with a significant risk of chronic kidney disease (CKD), especially within the first year. Substituting CNIs with mycophenolate mofetil monotherapy (MMF-MT) is a common strategy to mitigate this risk, particularly in patients with CKD, diabetes, or hypertension. Although some clinicians are cautious about converting patients with prior rejection episodes, others support switching to MMF-MT when patients have stable liver function and no recent rejection. In this study, the median time from LT to MMF-MT initiation was 72 months, and conversion was increasingly performed within 1–2 months with routine monitoring to detect rejection or side effects. Monitoring of mycophenolic acid (MPA) levels helped individualize MMF dosing and minimize toxicity, especially in high-volume centers where simpler monitoring methods are preferred over full AUC testing.
The long-term follow-up (median 78 months) showed significant improvement in kidney function, particularly in glomerular filtration rate (GFR) and serum creatinine, without a notable increase in rejection or adverse metabolic effects. Acute rejection post-MMF-MT occurred in only 7.4% of patients and was effectively managed. Adverse effects of MMF-MT were manageable, with only 2.5% requiring discontinuation. Patient survival at 5 years was 75.3%, aligning with existing literature. No significant differences were observed in treatment outcomes between earlier and later cohorts, and recurrence of biliary infections was prevented post-MMF-MT. Despite limitations like retrospective design and single-center data, this study supports MMF-MT as a safe and effective long-term alternative to CNIs in selected LT patients, emphasizing the importance of MPA level monitoring to balance efficacy and safety.
References
1. Charlton, M, Levitsky, J, Aqel, B, O´Grady, J, Hemibach, J, Rinella, M, et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation (2018) 102:727–43. doi:10.1097/TP.0000000000002147
2. Klupp, J, Pfitzmann, R, Langrehr, JM, and Neuhauss, P. Indications of Mycophenolate Mofetil in Liver Transplantation. Transplantation (2005) 80:S142–6. doi:10.1097/01.tp.0000187133.53916.8f
3. Morard, I, Mentha, G, Spahr, L, Majno, P, Hadengue, A, Huber, O, et al. Long-Term Renal Function After Liver Transplantation Is Related to Calcineurin Inhibitors Blood Levels. Clin Transpl (2006) 20:96–101. doi:10.1111/j.1399-0012.2005.00447.x
4. Ojo, AO. Renal Disease in Recipients of Nonrenal Solid Organ Transplantation. Semin Nephrol (2007) 27:498–507. doi:10.1016/j.semnephrol.2007.03.010
5. Kuo, HT, Sampaio, MS, Ye, X, Reddy, P, Martin, P, and Bunnapradist, S. Risk Factors for New-Onset Diabetes Mellitus in Adult Liver Transplant Recipients, an Analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing Database. Transplantation (2010) 89:1134–40. doi:10.1097/TP.0b013e3181d2fec1
6. Rodríguez-Perálvarez, M, Germani, G, Darius, T, Lerut, J, Tsochatzis, E, and Burroughs, AK. Tacrolimus Through Levels, Rejection and Renal Impairment in Liver Transplantation. A Systematic Review and Meta-Analysis. Am J Transpl (2012) 12:2797–814. doi:10.1111/j.1600-6143.2012.04140.x
7. Carenco, C, Assenat, E, Faure, S, Duny, Y, Danan, G, Bismuth, M, et al. Tacrolimus and the Risk of Solid Cancers After Liver Transplant: A Dose Effect Relationship. Am J Transpl (2015) 15:678–86. doi:10.1111/ajt.13018
8. Karie-Guigues, S, Janus, N, Saliba, F, Dumortier, J, Duvaux, C, Calmus, Y, et al. Long-Term Renal Function in Liver Transplantation Recipients and Impact of Immunosuppressive Regimens [Calcineurin Inhibitors Alone or in Combination With Mycophenolate Mofetil]:The TRY Study. Liver Transpl (2009) 15:1083–91. doi:10.1002/lt.21803
9. Hao, JC, Wang, WT, Yan, LN, Li, B, Wen, TF, Jang, JY, et al. Effect of Low-Dose Tacrolimus With Mycophenolate Mofetil on Renal Function Following Liver Transplantation. World J Gastroenterol (2014) 20:11356–62. doi:10.3748/wjg.v20.i32.11356
10. Duvoux, C, and Pageaux, GP. Immunosuppression in Liver Transplant Recipients With Renal Impairment. J Hepatol (2011) 54:1041–54. doi:10.1016/j.jhep.2010.12.001