Introduction
Kidney disease, both acute and chronic, is a major cause of illness and mortality in the world. Dysregulated immune responses are thought to have a key role in the pathophysiology of a number of renal disorders. Targeted treatments for many kidney illnesses are unavailable due to a lack of understanding of the underlying mechanisms. However, with recent progress in the development of organoids, new technology to model human tissues in vitro has become available.
Organoids, which represent a three-dimensional, multicellular culture system, generated from stem cells, closely match the structure and function of the organ. We highlight the potential applications of induced pluripotent stem cell (iPSC)-derived kidney organoids and tissues in the research of immune-mediated kidney disorders, as well as the function of the immune system in renal tissue formation, regeneration, and inflammation. We also discuss the problems of using organoids to accurately model immune-mediated kidney disorders, as well as the technical advancements that are still needed for the widespread use of this emerging technology in this context.
Organoids: the development of in vitro tissue models
Within the last 10 years organoids, three-dimensional structures generated from stem cells, which self-organize and differentiate into organ specific cell types, have emerged as an innovative in vitro model to study fundamental principles of human biology. Organoids furthermore offer unparalleled opportunities to investigate a wide range of pathologies and model disease of individual patients.
Organoids can be used as diagnostic tools or to test individual therapeutic options to tailor the treatment to a patient’s genotype and phenotype. At present, organoids can either be derived from embryonic stem cells, iPSCs, or organ specific ASCs. The development of iPSCs has been an important technical breakthrough, which provided an alternative to the use of blastocysts-derived embryonic stem cells. iPSCs are generated by reprogramming primary somatic cells, for example, fibroblasts, peripheral blood mononuclear cells (PBMCs), or urinary cells into embryonic stem cell-like cells. After transformation, iPSCs can be differentiated into precursor cells from all three germ layers.
ASC-derived organoids on the other hand are generated from isolated, tissue-specific cells. ASCs are cultured without reprogramming, and by the addition of carefully balanced Wnt-activators and tissue-specific growth factors, the cells are coaxed to form organoids. At present, ASC-derived organoid models exist for the intestine, liver, lung and multiple other organs. Recently, a similar approach for the generation of primary kidney tubular epithelial organoids called tubuloids was developed.
Today, organoids are employed in translational approaches for studying infectious diseases, cancer and hereditary diseases. They furthermore represent a promising technology for personalized and regenerative medicine.
iPSC-derived kidney organoids
The development of iPSC-derived kidney organoids
The development of organoids recapitulating the kidney’s individual multicellular architecture builds upon our understanding of nephrogenesis and formation of the three respective precursors pronephros, mesonephros, and metanephros during gestation. The complexity of the kidney with the nephron comprising of over 20 distinct cell types and the intricate reciprocal interactions of the metanephric mesenchyme and ureteric bud during nephrogenesis have delayed the development of kidney organoids.
In 2014, Taguchi and colleagues achieved the successful differentiation of human iPSCs into metanephric mesenchyme. Using phasic Wnt-stimulation and the timed addition of growth-factors mimicking physiological nephrogenesis, they induced metanephric progenitors to give rise to kidney tubules and glomeruli.
This resulted in the formation of intricate, multicellular kidney organoids containing nephron-like structures with an associated collecting duct network surrounded by renal interstitium and endothelial cells. These approaches are however costly and time-consuming; therefore, more recently efforts have been made to increase the scalability and reduce costs for the generation of iPSC-derived kidney organoids.
iPSC-derived kidney organoids: characterization and assessment
iPSC-derived kidney organoids have been characterized to assess their value for disease modeling, drug- and toxicity-screening, and a new tool to further advance personalized medicine. Especially the fidelity of iPSC-derived kidney organoids to robustly replicate normal kidney development with particular attention paid to cellular identity, and development has been elaborately investigated.
Applying single-cell RNA-sequencing approaches revealed a high level of congruence between kidney organoids and the human fetal kidney regarding gene expression within endothelial, stromal, and nephron cell types. Further, distinct nephron segments were identified by protein expression and included cells with profiles of podocytes, early proximal tubule cells, early distal tubule cells, and collecting duct epithelial cells.
Podocytes in kidney organoids show appropriate basolateral polarity and gene expression profile better reflecting the in vivo situation. There was evidence for cubilin-mediated endocytosis in proximal tubule cells, which supports an advanced functional cell maturation. The potential for nephrotoxicity screening was tested with cisplatin, which induced dose-dependent apoptosis in proximal tubular cells mirroring the effect in vivo.
iPSC-derived kidney organoids: challenges and adaptions
One of the most critical limitations of kidney organoids is the fact that they recapitulate the developing human fetal kidney. Evidence for the presence of cell-type specific ligands and receptors mediating human nephrogenesis as well as markers of terminal differentiation are still missing.
An additional limitation of iPSC-derived kidney organoids is the absence of certain cell types, which are present in vivo in the human kidney. Although there is evidence for vasculature formation and development of endothelial networks in iPSC-derived kidney organoids, these models do not form a fully matured glomerulus. Efforts have been made to establish a functioning blood supply to kidney organoids. iPSC-derived kidney organoids were transplanted under the renal capsule of immune-compromised mice. After transplantation, the organoids grew significantly in size while maintaining glomerular and tubular structures.
The transplanted organoids formed a glomerular basement membrane and the tubule epithelium formed a single monolayer with a well-developed apical brush border and luminal microvilli and cilia. Kidney organoids exposed to high fluidic shear stress developed a mature vasculature than organoids cultured under static or low fluidic shear stress conditions. Altogether, the exposure to controlled flow of fluids facilitated the formation of vasculature and enhanced the maturity of tubular epithelium.
iPSCs are vulnerable to tumorigenicity and the removal of epigenetic marks by reprogramming impedes their use for non-heritable kidney diseases and related personalized medicine.
Tubuloids
Tubuloids: establishment, characterization, and assessment
Renal tubular epithelial organoids are termed as tubuloids as they represent distinct tubule segments of the nephron but lack glomerular cells. The renal epithelial cells required to generate tubuloids are obtained from kidney tissue or urine. Especially use of exfoliated renal epithelial cells from urine is a promising non-invasive technique for organoid generation.
The protocol harnesses the kidney’s mechanisms for tissue repair and regeneration by promoting the dedifferentiation of tubular epithelial cells into a progenitor state as well as proliferation of progenitor cells into segment specific tubule epithelium. Yet, based on single-cell RNA-sequencing analyses, gene expression profiles of distinct nephron segments were detected including the proximal tubule, loop of Henle, distal tubule, and collecting duct. Further, tubuloids contained cells with multilineage potential as tubuloid lines established from a single-cell expressed marker genes of different nephron segments.
Tubuloids: challenges and adaptions
Although tubuloids have the advantage to be derived from ASCs, the absence of stromal populations, podocytes, and vascularization limits their use for modelling diseases in which these renal structures play an important role, e.g., glomerulonephritis. At present, tubuloid culture protocols are optimized to generate proximal tubule cells. Adaptations of the protocols are required to allow the formation of tubuloids representing the in vivo distribution of all distinct nephron segments with their associated transporter proteins and enzymes.
Under these conditions, tubuloids formed leak-tight, perfusable, differentiated kidney tubules. This approach facilitates the engineering of more complex tissue structures. Further, the exposure to fluid flow enables continuous media refreshment of tubuloid cultures. Building on these recent developments will allow the generation of more complex tubuloid models.
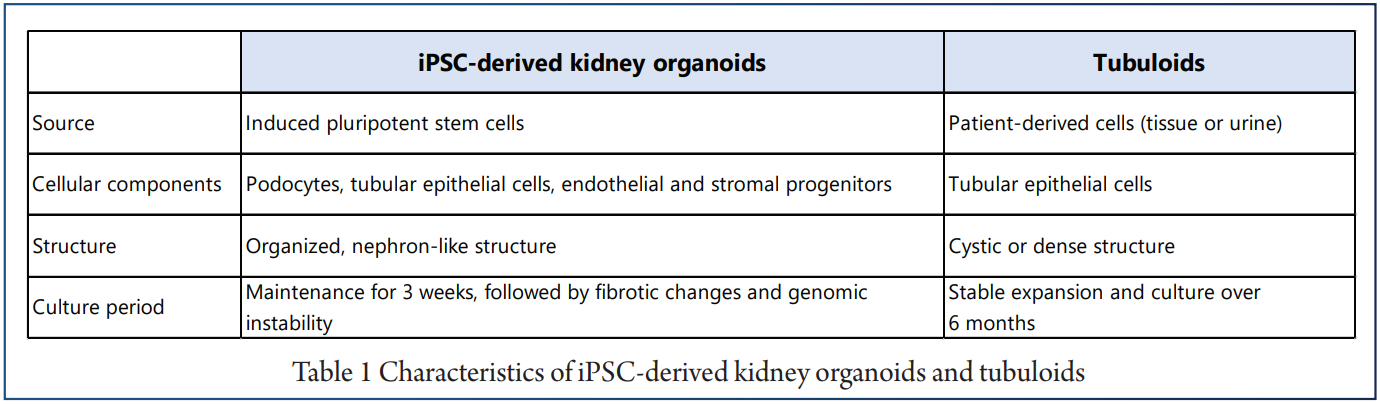
The main differences between the iPSC-derived organoids and tubuloids are that iPSC-derived kidney organoids recapitulate nephrogenesis, whereas tubuloids model renal tissue regeneration and repair of the renal tubule.
Use of organoid models beyond stem cell biology
In recent publications, kidney organoids and tubuloids were primarily used to assess developmental and genetic diseases. To advance organoid models, protocols for the co-culture of organoids and immune cells have been developed. Recently, it has been shown that tumor-reactive CD8+ T cells can be expanded when co-cultured with tumor organoids and assessed for their anti-tumor responses, providing proof-of-concept for developing patient-specific T cell-based therapies.
iPSC-derived kidney organoids and tubuloids: unique tissue models
For the successful employment of organoids to investigate immune-mediated kidney diseases, the advantages Further, the exposure to fluid flow enables continuous and drawbacks of iPSC-derived kidney organoids and media refreshment of tubuloid cultures. Building on tubuloids need to be assessed to design meaningful these recent developments will allow the generation of studies (Table 1).
Similarly, tumor organoid–T cell co-cultures could be used to assess the efficacy of individual drugs in the presence of immune cells to tailor treatment to the individual patient for example in children suffering from kidney cancer. Further, the severe acute respiratory syndrome corona virus type 2 (SARS-CoV-2) pandemic has underlined the value of kidney organoids in the field of infectious diseases research, as kidney organoids expressing ACE2 (angiotensin converting enzyme 2) were used as in vitro models for SARS-CoV-2 infection.
The addition of human recombinant soluble ACE2 reduced viral infection of kidney organoids and blocked early stages of infection, demonstrating the importance of ACE2 for infection of SARS-CoV-2. Tubuloids have furthermore been infected with BK virus, a member of the polyoma-virus family, which resulted in intranuclear basophilic viral inclusions as seen in BK nephropathy.
Modeling immune-mediated kidney diseases with kidney organoids and tubuloids
Rapid progressive glomerulonephritis
The most aggressive form of glomerular inflammation is seen in rapid progressive glomerulonephritis. CD4+ T cells have been shown to drive rapid progressive glomerulonephritis as seen in ANCA-associated glomerulonephritis, anti-GBM nephritis, and lupus nephritis. Especially the role of IL-17 producing CD4+ TH17 cells has gained increasing attention in kidney inflammation. An increased TH17 response is associated with a more severe course of glomerulonephritis and tubulointerstitial injury.
IL-17C deficiency as well as deficiency of the respective IL-17 receptor E furthermore ameliorates tissue injury and kidney function in mouse models of crescentic glomerulonephritis and lupus nephritis. Both iPSC-derived kidney organoids and tubuloids furthermore provide a human cell-based system to determine T helper cell polarization by renal epithelial cells in kidney diseases even at the individual patient level.
Lupus nephritis
Approximately 50% of SLE patients develop lupus nephritis, which is associated with the dysregulation of several TLRs (toll-like receptors) including TLR4, TLR7, and TLR9. Podocytes and renal tubular epithelial cells constitutively express TLRs and are able to recognize pathogen-associated molecular patterns (PAMPs) of bacteria or viruses. Activation of TLRs in podocytes and renal tubular epithelial cells induces the production of proinflammatory chemokines and cytokines, which attract and activate immune cells including T and B cells. Dysregulated T and B cell responses importantly contribute to the pathogenesis of lupus nephritis. Therefore, podocytes and tubular epithelial cells may play an important role in the activation of the adaptive arm of the immune system, in particular upon kidney injury.
IgA nephropathy
IgA nephropathy is the most common form of primary glomerulonephritis, characterized by circulating immune complexes that are trapped in the filtration barrier. Critical in the pathogenesis of IgA nephropathy is galactose-deficient IgA1, which elicits an autoantibody response against N-acetylgalactosamine epitopes on galactose-deficient IgA1 and formation of immune complexes. The deposition of immune complexes further activates the complement system, and induces cytokine and chemokine production by mesangial cells. Studies revealed that the transferrin receptor (CD71) can function as mesangial cell IgA receptor and facilitates immune complex deposition.
Glomerular antibody deposition
Antibody deposition at the glomerular basement membrane can result in membranous nephropathy that is characterized by high proteinuria and a granular pattern of glomerular immune deposits. Autoantibodies against antigens expressed by healthy podocytes including the PLA2R (M-type phospholipase A2 receptor) or Thrombospondin type-1 domain containing 7 are detected in the majority of patients with membranous nephropathy.
In anti-GBM nephritis, autoantibodies bind to mainly two epitopes on the α3 chain of the non-collagenous-1 (NC1) domain of type IV collagen and form linear IgG deposits along the GBM causing rapidly progressive glomerulonephritis. Studies assessing the expression of these antigens in iPSC-derived kidney organoids could provide a new platform to dissect the antibody-mediated and immune cell-mediated mechanisms.
Interstitial nephritis and modelling kidney injury with organoids and tubuloids
In addition to the activation of inflammatory pathways by PAMPs, tubular epithelial cells are able to secret a range of proinflammatory cytokines upon ischemia further activating neighbouring immune cells. Tubuloids have recently been plated in a “organ-on-a-chip” platform, which allows medium perfusion of the leak-tight tubes.
Tubuloids could be used to further determine the effects of crystals on epithelial cells and reciprocal effects for crystal retention as well as progression to nephrolithiasis. Even after removal of the drug, many patients do not recover their baseline kidney function. iPSC-derived kidney organoid and tubuloid models established from patients and co-cultured with autologous T cells could be used to assess the effect of steroids and other anti-inflammatory drugs on tubular regeneration.
Nephrotoxicity of drugs including gentamicin and cisplatin has already been successfully modelled in kidney organoids, which supports their utility for drug-testing in a patient-specific in vitro model.
Kidney organoids and tubuloids to model immune-mediated renal tissue regeneration
In the last years, the immune system’s role in tissue repair mechanisms is increasingly appreciated. These studies revealed that TNFα produced by fetal intestinal CD69+ CD4+ T effector memory cells promotes epithelial stem cell proliferation and aids the development of the fetal intestine, but can also mediate intestinal inflammation upon premature birth. This study illustrates that organoid-immune cell co-cultures can be used to model inflammatory diseases and immune-mediated instruction of fetal tissue development.
Similarly, iPSC-derived kidney organoids recapitulating nephrogenesis could be used to study the influence of immune cell-derived cytokines on kidney tissue development. Furthermore, as tubular cells are primarily affected in acute kidney injury, co-cultures of tubuloids with CD4+ T cells would allow to investigate their role in facilitating tissue regeneration and repair.
Current limitations of organoid-immune cell co-cultures
At present, several challenges that impede the co-culture of iPSC-derived kidney organoids and tubuloids with immune cells exist. Foremost, the propagation of iPSC-derived kidney organoids and tubuloids requires a complex medium enriched with growth factors and nutrients . Immune cells can exhibit receptors for these medium components, which influence their activation and differentiation.
On the other hand, immune cell cultivation also requires certain media components, which in return might impact organoid cells. IL-2 is an important component of T cell culture medium. A recent study revealed that podocytes also express a functional IL-2 receptor, whose activation results in podocyte injury, apoptosis, and mitochondrial depolarization.
In sum, the application of kidney organoids and tubuloids in co-cultures with immune cells requires careful assessment of media components to rule out possible bias and design meaningful studies.
Conclusion
Immune-mediated diseases are characterised by complex interactions between epithelial cells and immune system cells. Both iPSC-derived kidney organoids and tubuloids, which were recently created, have the potential to be used in future studies on immune-mediated kidney disorders. iPSC-derived kidney organoids are ideal models for properly representing several elements of the growing human kidney, allowing researchers to learn more about nephrogenesis and congenital abnormalities. To enhance these models to resemble adult kidney tissue, more development of iPSC-derived kidney organoids is necessary.
Tubuloids, on the other hand, have the potential for patient-specific disease modelling and personalised treatment due to their quick creation and genetic stability, expressing the donor’s genotype and phenotype throughout long culture periods. New perspectives for simulating immune-mediated kidney disorders have emerged as a result of recent advancements in the co-culture of organoids and immune cells.
The innovations needed to establish robust kidney organoid-immune cell co-culture systems can be carried ahead by building on recent advances made within other organoid systems. Finally, iPSC-derived kidney organoids and tubuloids offer a unique platform for research into immune-mediated kidney development, tissue regeneration, and inflammation.
References
1. Allam R, Scherbaum CR, Darisipudi MN et al (2012) Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 23:13751388.
2. Almaani S, Meara A, Rovin BH (2017) Update on Lupus Nephritis. Clin J Am Soc Nephrol 12:825 LP – 835.
3. Banas MC, Banas B, Hudkins KL et al (2008) TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19:704–713.
4. Banu N, Meyers CM (1999) IFN-γ and LPS differentially modulate class II MHC and B7–1 expression on murine renal tubular epithelial cells. Kidney Int 55:2250–2263.
5. Bar-Ephraim YE, Kretzschmar K, Clevers H (2020) Organoids in immunological research. Nat Rev Immunol 20:279–293.
6. Beck LH, Bonegio RGB, Lambeau G et al (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361:11–21.
7. Berger K, Bangen JM, Hammerich L et al (2014) Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci 111:1533 LP – 1538.
8. Berthoux F, Suzuki H, Thibaudin L et al (2012) Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23:1579 LP – 1587.
9. Bishop GA, Waugh JA, Hall BM (1988) Expression of hla antigens on renal tubular cells in culture: II. Effect of increased hla antigen expression on tubular cell stimulation of lymphocyte activation and on their vulnerability to cell-mediated lysis. Transplantation 46:303–310.
10. Biton M, Haber AL, Rogel N et al (2018) T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175:1307–1320.e22. 11. Bohle A (1994) On the pathogenesis of chronic renal failure in primary glomerulopathies: A view from the interstitium. Exp Nephrol 2:205–210
12. Braun F, Homeyer I, Alachkar N, Huber TB (2021) Immune-mediated entities of (primary) focal segmental glomerulosclerosis. Cell Tissue Res.
13. Calandrini C, Schutgens F, Oka R et al (2020) An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun 11:1310.