Introduction
Kidney transplantation (KTx) is the preferred treatment for children with stage 5 chronic kidney disease. Potent immunosuppressants such as tacrolimus (Tac), a calcineurin inhibitor (CNI), and mycophenolate mofetil (MMF), as well as anti-infective prophylaxis, have significantly improved short-term transplant outcomes. Long-term graft survival is, however, limited. While CNI-induced nephrotoxicity was previously thought to be the most common cause of late graft function deterioration, new evidence suggests that the causes are multifactorial and complex. Alloantigen-dependent factors, particularly de novo donor-specific antibodies (dnDSA) directed against human leukocyte antigens (HLA), play an important role in this context. Tacrolimus (Tac) intraindividual variability (TacIPV) in pediatric kidney transplant patients is only poorly understood. We investigated the impact of TacIPV on de novo donor-specific HLA antibodies (dnDSA) development and allograft rejection in Caucasian pediatric recipients of a living or deceased donor kidney with low immunological risk.
Methods
This was a single-center retrospective study including 48 pediatric kidney transplant recipients. TacIPV was calculated based on the coefficient of variation (CV%) 6–12 months posttransplant. TacIPV cutoff was set at the median (25%). Outcome parameters were dnDSA development and rejection episodes.
Results
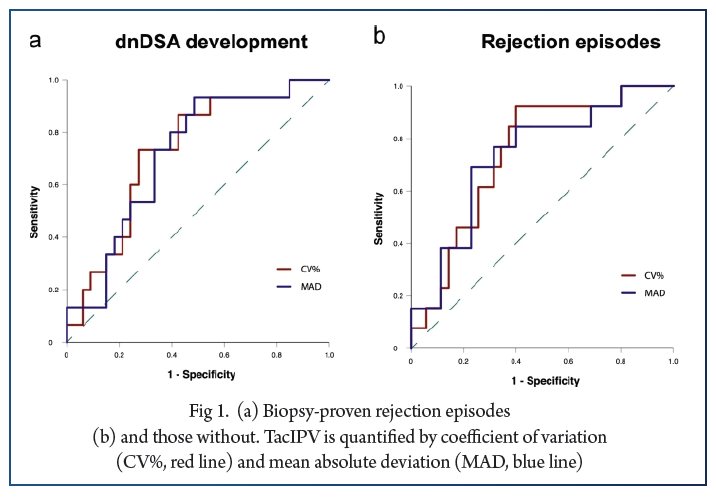
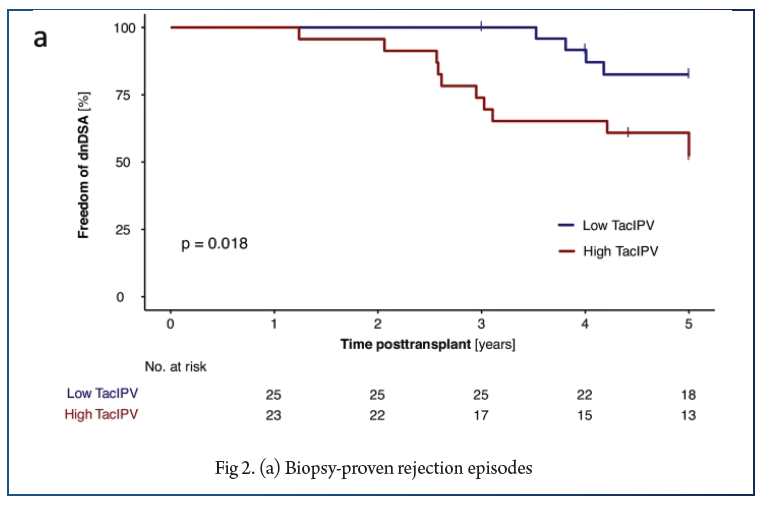
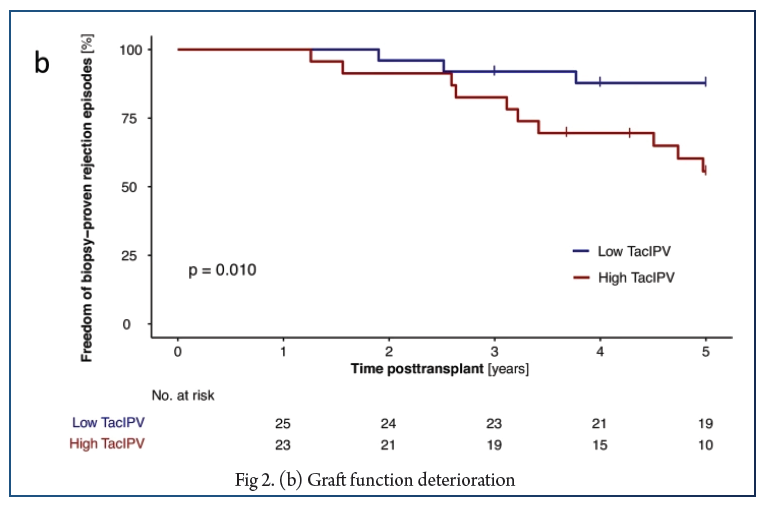
In total, 566 Tac levels were measured with median 11.0 (6.0–17.0) measurements per patient. The cutoff of 25% corresponded to the median CV% in our study cohort (25%, IQR 18–35%) and was comparable to cutoffs determined by receiver operating characteristic (ROC) curve analysis. High TacIPV was associated with higher risk of dnDSA development (HR 3.4, 95% CI 1.0–11.1, P = 0.047; Kaplan–Meier analysis P = 0.018) and any kind of rejection episodes (HR 4.1, 95% CI 1.1–14.8, P = 0.033; Kaplan–Meier analysis P = 0.010). There was a clear trend towards higher TacIPV below the age of 6 years. TacIPV (CV%) was stable over time. A TacIPV (CV%) cutoff of 30% or IPV quantification by mean absolute deviation (MAD) showed comparable results.
Conclusions
High TacIPV is associated with an increased risk of dnDSA development and rejection episodes > year 1 posttransplant even in patients with low immunological risk profile. Therefore, in patients with high TacIPV, potential causes should be addressed, and if not resolved, changes in immunosuppressive therapy should be considered.
References:
1. Gondos A, Döhler B, Brenner H, Opelz G (2013) Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation 95:267–274. https://doi.org/10.1097/TP.0b013e3182708ea8.
2. Neuberger JM, Bechstein WO, Kuypers DRJ, Burra P et al (2017) Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) group. Transplantation 101:S1–S56. https://doi.org/10.1097/TP.0000000000001651.
3. Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M et al (2012) Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant: clinical pathologic correlations of de novo DSA. Am J Transplant 12:1157–1167. https://doi.org/10.1111/j.1600-6143.2012.04013.x.
4. Loupy A, Hill GS, Jordan SC (2012) The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8:348–357. https://doi.org/10.1038/nrneph.2012.81.
5. Sellarés J, de Freitas DG, Mengel M, Reeve J et al (2012) Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12:388–399. https://doi.org/10.1111/j.1600-6143.2011.03840.x.
6. Mendoza Rojas A, Hesselink DA, van Besouw NM, Baan C et al (2019) Impact of low tacrolimus exposure and high tacrolimus intra-patient variability on the development of de novo anti-HLA donor-specific antibodies in kidney transplant recipients. Expert Rev Clin Immunol 15:1323–1331. https://doi.org/10.1080/1744666X.2020.1693263.
7. Shuker N, van Gelder T, Hesselink DA (2015) Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev 29:78–84. https://doi.org/10.1016/j.trre.2015.01.002.
8. Gonzales HM, McGillicuddy JW, Rohan V, Chandler J et al (2020) A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am J Transplant 20:1969–1983. https://doi.org/10.1111/ajt.16002.
9. Borra LCP, Roodnat JI, Kal JA, Mathot R et al (2010) High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 25:2757–2763. https://doi.org/10.1093/ndt/gfq096.
10. Sapir-Pichhadze R, Wang Y, Famure O, Li Y et al (2014) Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int 85:1404–1411. https://doi.org/10.1038/ki.2013.465.
11. Rodrigo E, Segundo DS, Fernández-Fresnedo G, López-Hoyos M et al (2016) Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation 100:2479–2485. https://doi.org/10.1097/TP.0000000000001040.
12. Sablik KA, Clahsen-van Groningen MC, Hesselink DA, van Gelder T et al (2018) Tacrolimus intra-patient variability is not associated with chronic active antibody mediated rejection. PLoS One 13:e0196552. https://doi.org/10.1371/journal.pone.0196552.
13. Vanhove T, Vermeulen T, Annaert P, Lerut E et al (2016) High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am J Transplant 16:2954–2963. https://doi.org/10.1111/ajt.13803.
14. Solomon S, Colovai A, Del Rio M, Hayde N (2020) Tacrolimus variability is associated with de novo donor-specific antibody development in pediatric renal transplant recipients. Pediatr Nephrol 35:261–270. https://doi.org/10.1007/s00467-019-04377-6.
15. Pizzo HP, Ettenger RB, Gjertson DW, Reed E et al (2016) Sirolimus and tacrolimus coefficient of variation is associated with rejection, donor-specific antibodies, and nonadherence. Pediatr Nephrol 31:2345–2352. https://doi.org/10.1007/s00467-016-3422-5.
16. Kaya Aksoy G, Comak E, Koyun M, Akbas H et al (2019) Tacrolimus variability: a cause of donor-specific anti-HLA antibody formation in children. Eur J Drug Metab Pharmacokinet 44:539–548. https://doi.org/10.1007/s13318-019-00544-0.
17. Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand A et al (2010) Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant 14:968–975. https://doi.org/10.1111/j.1399-3046.2010.01409.x.
18. Fichtner A, Süsal C, Höcker B, Rieger S et al (2021) Association of non-HLA antibodies against endothelial targets and donor-specific HLA antibodies with antibody-mediated rejection and graft function in pediatric kidney transplant recipients. Pediatr Nephrol. https://doi.org/10.1007/s00467-021-04969-1.
19. Süsal C, Wettstein D, Döhler B, Morath M et al (2015) Association of kidney graft loss with de novo produced donor-specific and non-donor-specific HLA antibodies detected by single antigen testing. Transplantation 99:1976–1980. https://doi.org/10.1097/TP.0000000000000672.