Introduction
Rheumatoid arthritis is an immune disorder that causes joint inflammation and damage, often leading to deformities and disability. Conventional treatments have limitations, but the emergence of antibody drugs offers promise by targeting specific cytokines like IL-1, IL-6, and TNF-α. This approach can reduce side effects and improve therapeutic outcomes, especially when combined with drug delivery nanosystems. However, challenges remain in their clinical use, such as patient compliance and immunosuppression. The article provides an overview of RA pathogenesis, available antibody drugs, and DDSs, and proposes strategies to address these limitations. Overall, the article is optimistic about the potential of antibody drugs and DDSs to effectively treat RA.
Therapeutic approaches
Rheumatoid arthritis (RA) is treated clinically using surgery and drug therapy. Surgery may cause secondary joint cavity injury and compromise joint function. On the other hand, drug therapy is less invasive and offers a broad range of options. The drugs used for RA treatment include disease-modifying anti-rheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), and antibody drugs. DMARDs have low bioavailability and poor specificity, posing a significant risk to patient health. NSAIDs have limited effectiveness and serious side effects. GCs exhibit good immunosuppressive effects but have long-term safety concerns. In contrast, antibody drugs have fewer side effects, high specificity for their target sites, and broad applications in treating RA. Antibody drugs are a safe, effective, and reliable treatment for RA. They eliminate high-level immune complexes, inhibit inflammatory factors, and alleviate damage to cartilage and synovium, leading to symptom improvement and pain relief. Antibody drugs offer several advantages over other treatment options, including better safety profiles, superior efficacy, and clear biological pharmacological mechanisms. Overall, antibody drugs represent a promising approach to treating RA.

Antibody drugs for the treatment of RA
With the continuous development of molecular biology, we have gained a deeper understanding of the pathogenesis and etiology of RA. In addition, the continuous progress and maturity of biochemistry and nanotechnology have greatly increased the potential application of antibody drugs. Currently, approved antibody drugs are classified into several types based on their mechanisms of action, including TNF-α inhibitors, interleukin 1 inhibitors, interleukin 6 inhibitors, CD80/86-CD 28 inhibitors, and B-cell eliminating antibodies. Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that plays a crucial role in the etiology of rheumatoid arthritis (RA) by mediating synovial hyperplasia and generating other proinflammatory factors. TNF-α inhibitors, including etanercept, infliximab, adalimumab, certolizumab, and golimumab, are the most widely used biological drugs to treat RA. While all anti-TNF-α drugs can competitively bind to TNF-α receptors on the cell surface and inhibit TNF-α biological activity, they differ in their molecular structures and administration schemes. Etanercept was the first TNF-α inhibitor discovered, and its clinical efficacy and safety have been confirmed in early clinical trials. However, long-term injection of etanercept may induce targeted toxicity, including severe infection, and increase the risk of malignancy and tuberculosis during the course of RA treatment. Interleukin-1 (IL-1) is a group of cytokines associated with inflammation and the innate immune response. While cytokines such as IL-1α, IL-1β, and IL-33 exhibit proinflammatory activity, cytokines such as IL-1Ra, IL-37, and IL-38 demonstrate anti-inflammatory activity. IL-1 and its receptor antagonist (IL-1Ra) play an essential role in the development of RA. Anakinra is a recombinant human IL-1Ra that blocks IL-1α and IL-1β activity by competitively inhibiting their binding to the IL-1 receptor, resulting in anti-inflammatory and anti-arthritic effects. Anakinra has been approved for the treatment of RA and other inflammatory diseases. However, its clinical efficacy is limited, and it may cause injection site reactions, infections, and other adverse effects. Additionally, other IL-1 inhibitors, such as canakinumab and rilonacept, have been developed and are under investigation for the treatment of RA.
Means to improve the efficacy of monoclonal antibody drugs
Gold nanoparticles (GNPs)
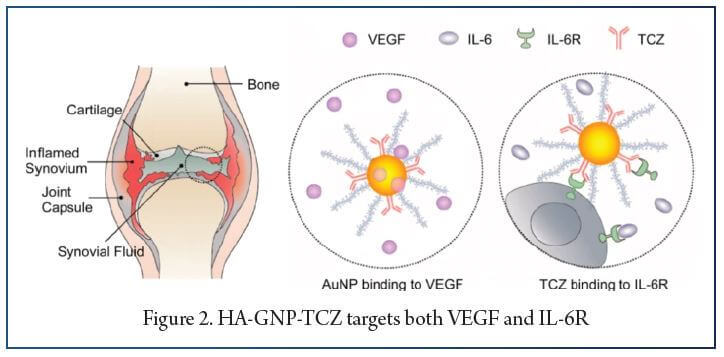
Gold nanoparticles (GNPs) are gaining popularity in drug delivery due to their stability, effectiveness, biocompatibility, and modifiable optical properties. They can be prepared in various sizes and shapes, exhibit good targeting performance, and have a high drug loading capability. GNPs can preferentially absorb X-rays to enhance the effect of radiation therapy and can also be used as nanoprobes and contrast agents for diagnosing rheumatoid arthritis (RA). Studies have shown that GNPs have anti-angiogenic effects, reduce inflammation levels, and inhibit bone erosion or cartilage destruction in RA. Researchers have designed a new drug delivery system, HA-GNP-TCZ, which can target both IL-6 and VEGF and significantly reduce the level of inflammatory cell infiltration, cartilage destruction, and bone erosion in RA. GNPs accumulate in different organs but do not cause any toxicity or cell damage and can effectively delay the progression of RA. The application of GNPs in the biomedical field holds great potential, and targeted treatment of TCZ delivered by GNPs has shown promising results in alleviating the narrowing of the joint space, bone erosion, and inflammation in RA patients.
Albumin nanoparticles
Albumin is a highly abundant protein in human plasma that plays an important role in maintaining plasma osmotic pressure, detoxifying the body, and acting as an important nutrient. Studies have shown that albumin nanoparticles can be used as effective drug carriers to target inflammatory joints in rheumatoid arthritis (RA) patients, due to the increased demand for albumin in these joints. Furthermore, albumin has high affinity for the protein SPARC, which is overexpressed in inflammatory joints and contributes to the active targeting of albumin nanoparticles. Researchers have also developed a recombinant protein by fusing human serum albumin (HSA) to the carboxyl terminus of IL-1ra, which exhibited a longer serum half-life and excellent targeting performance. This fusion protein demonstrated a significant therapeutic effect in joint inflammation, with a lower distribution rate in other parts of the body. In conclusion, albumin nanoparticles and albumin-based fusion proteins are promising drug delivery systems for the treatment of RA, due to their excellent targeting ability, prolonged drug action, and improved pharmacokinetic properties and efficacy.
Dendrimers
Dendrimers are synthetic polymers with a dendritic structure that can bind to antibodies due to their large surface structure. They have good uniformity, high biocompatibility, and well-defined structure. Common dendrimer skeletons include polyamidoamine dendrimers (PAMAM), polypropylene imine (PPI), polyesters, and scaffolds containing phosphorus and silicon atoms in the structure. Dendrimers have been used as nanoplatforms for drugs, nucleic acid transporters, contrast agents, etc. PAMAM can be modified with chondroitin sulfate (CS) and anti-TNF RA antibodies to increase affinity with cartilage, showing good cytocompatibility and hemocompatibility. Carbon silane dendrimers have also attracted considerable attention due to their excellent hydrophobicity.
Nanogels
Nano-gels are polymeric gels that exist as nanoparticles and can respond to different environmental stimuli. They have advantages such as high stability, good biocompatibility, and can be used as drug carriers. They can be used topically to reduce adverse reactions and are able to penetrate the skin to produce an anti-inflammatory effect. Nanogels also have a high loading capacity for protein drugs and can be used to improve their biological half-life.
Others
Temperature-sensitive drug delivery systems have gained attention for improving drug stability and prolonging pharmacokinetic parameters. Jung et al. developed a temperature-modulated noncovalent interaction controllable complex that improved serum stability and therapeutic effect in vivo. Microneedle systems have also been developed as a transdermal drug delivery method for improved patient compliance. Cao et al. developed a hyaluronic acid crosslinked microneedle system to deliver etanercept with good anti-inflammatory effects and joint protection. The microneedle system showed similar efficacy to classic subcutaneous administration with higher biocompatibility and compliance.
Summary
Rheumatoid arthritis (RA) is an autoimmune disease causing inflammation, cartilage and bone erosion, joint swelling, and pain, ultimately affecting patient survival and lifespan. Antibody drugs represent a new choice for RA treatment; however, patient compliance and immunogenicity are challenges. Transdermal delivery nanosystems improve patient compliance by avoiding injections and pain. Nanoparticles capable of carrying a payload reduce immunogenicity and nonspecific immunosuppression. Active targeted nanodevices could aggregate drugs in the lesion to avoid systemic exposure and potential infections and cancers, particularly tuberculosis.
References:
1. Shams S, Martinez JM, Dawson JRD, et al. The Therapeutic Landscape of Rheumatoid Arthritis: current state and future directions. Front Pharmacol. 2021;12:680043. https://doi.org/10.3389/fphar.2021.680043. Published 2021 May 28.
2. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328–37. https://- doi.org/10.1016/S0140-6736(17)31472-103.
3. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72. https://doi.org/10.1001/jama.2018.13103.
4. Alam J, Jantan I, Bukhari SNA. Rheumatoid arthritis: recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother. 2017;92:615–33. https://doi.org/10.1016/j.biopha.2017.05.055.
5. Wang Q, Qin X, Fang J, Sun X. Nanomedicines for the treatment of rheumatoid arthritis: state of art and potential therapeutic strategies. Acta Pharm Sin B. 2021;11(5):1158–74. https://doi.org/10.1016/j.apsb.2021.03.013.
6. Kondo Y, Yokosawa M, Kaneko S, et al. Review: transcriptional regulation of CD4 + T-Cell differentiation in experimentally Induced Arthritis and Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70(5):653–61. https://doi.org/10.1002/art.40398.
7. Matsuo Y, Saito T, Yamamoto A, Kohsaka H. Origins of fibroblasts in rheumatoid synovial tissues: implications from organ fibrotic models. Mod Rheumatol. 2018;28(4):579–82. https://doi.org/10.1080/14397595.2017.1386837.
8. Chuang SY, Lin CH, Huang TH, Fang JY. Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials (Basel). 2018;8(1):42. Published 2018 Jan 15. doi:https://doi.org/10.3390/nano8010042.
9. Guo L, Zhong S, Liu P, Guo M, Ding J, Zhou W. Radicals scavenging MOFs enabling targeting delivery of siRNA for rheumatoid arthritis therapy. Small. 2022;18(27):e2202604. https://doi.org/10.1002/smll.202202604.
10. Yang Y, Guo L, Wang Z, et al. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and repolarization. Biomaterials. 2021;264:120390. https://doi.org/10.1016/j.biomaterials.2020.120390.
11. Chen SJ, Lin GJ, Chen JW et al. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int J Mol Sci. 2019;20(6):1332. Published 2019 Mar 16. doi:https://doi.org/10.3390/ijms20061332.
12. raki Y, Mimura T. Matrix Metalloproteinase Gene Activation resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int J Mol Sci. 2017;18(5):905. https://doi.org/10.3390/ijms18050905. Published 2017 Apr 25.
13. Arioka M, Takahashi-Yanaga F. Glycogen synthase kinase-3 inhibitor as a multitargeting anti-rheumatoid drug. Biochem Pharmacol. 2019;165:207–13. https://- doi.org/10.1016/j.bcp.2019.02.020.
14. Tanaka S, Tanaka Y, Ishiguro N, Yamanaka H, Takeuchi T. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol. 2018;28(1):9–16. https://doi.org/10.1080/14397595.2017.1369491.
15. Küçükdeveci AA. Nonpharmacological treatment in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33(5):101482. https://- doi.org/10.1016/j.berh.2019.101482.
16. Köhler BM, Günther J, Kaudewitz D, Lorenz HM. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8(7):938. https://- doi.org/10.3390/jcm8070938. Published 2019 Jun 28.
17. XL, Lu KJ, Yao XQ, Ying XY, Du YZ. Stimuli-responsive drug Delivery Systems as an emerging platform for treatment of rheumatoid arthritis. Curr Pharm Des. 2019;25(2):155–65. https://doi.org/10.2174/1381612825666190321104424.